Product Info Summary
| SKU: | EK0921 |
|---|---|
| Size: | 96 wells/kit, with removable strips. |
| Reactive Species: | Human |
| Application: | ELISA |
| Sample Types: | cell culture supernatants, cell lysates, serum and plasma (heparin, EDTA). |
Product info
Product Name
Human TNFSF13/APRIL ELISA Kit PicoKine®
View all APRIL/TNFSF13 ELISA kits
SKU/Catalog Number
EK0921
Size
96 wells/kit, with removable strips.
*Question: How many samples can I assay/run in this kit?
Description
Human TNFSF13/APRIL ELISA Kit PicoKine® (96 Tests). Quantitate Human TNFSF13 in cell culture supernatants, cell lysates, serum and plasma (heparin, EDTA). Sensitivity: 10pg/ml. The brand Picokine indicates this is a premium quality ELISA kit. Each Picokine kit delivers precise quantification, high sensitivity, and excellent reproducibility. Only our most reliable and effective kits qualify as Picokine, guaranteeing top-tier results for your assays.
Storage & Handling
Store at 4°C for 6 months, at -20°C for 12 months. Avoid multiple freeze-thaw cycles (Ships with gel ice, can store for up to 3 days in room temperature. Freeze upon receiving.)
Cite This Product
Human TNFSF13/APRIL ELISA Kit PicoKine® (Boster Biological Technology, Pleasanton CA, USA, Catalog # EK0921)
Clonality of Antibodies
See Datasheet for details
Standard Protein
Expression system for standard: NS0; Immunogen sequence: A105-L250
Sensitivity
<10 pg/ml
Assay Range
156 pg/ml - 10,000 pg/ml
Standard Dilution Instructions

See datasheet of EK0921 for more details
Cross-reactivity
There is no detectable cross-reactivity with other relevant proteins.
Reactive Species
EK0921 is reactive to TNFSF13 in Human samples
Validated Sample Types
cell culture supernatants, cell lysates, serum and plasma (heparin, EDTA).
Application Guarantee
EK0921 is guaranteed for ELISA in Human by Boster Guarantee
See how Boster Bio validate our ELISA kits: ELISA Validation Information
Background of APRIL/TNFSF13
Tumor necrosis factor ligand superfamily member 13 (TNFSF13) also known as a proliferation-inducing ligand (APRIL) is a protein that in humans is encoded by the TNFSF13 gene. TNFSF13 has also been designated CD256 (cluster of differentiation 256). The protein encoded by this gene is a member of the tumor necrosis factor ligand (TNF) ligand family. This protein is a ligand for TNFRSF17/BCMA, a member of the TNF receptor family. This protein and its receptor are both found to be important for B cell development. In vivo experiments suggest an important role for APRIL in the long-term survival of plasma cells in the bone marrow. Mice deficient in April showed a reduced ability to support plasma cell survival In vitro experiments suggested that this protein may be able to induce apoptosis through its interaction with other TNF receptor family proteins such asTNFRSF6/FAS and TNFRSF14/HVEM. Three alternatively spliced transcript variants of this gene encoding distinct isoforms have been reported. The TNFSF13 gene lies 878 bp downstream of the TNFSF12 gene on chromosome 17p13.1.
Kit Components
| Catalog Number | Description | Quantity |
|---|---|---|
| EK0921-CAP | Anti-Human TNFSF13 Pre-coated 96-well strip microplate | 1 |
| EK0921-ST | Human TNFSF13 Standard | 2 vials, 10 ng/tube |
| EK0921-DA | Human TNFSF13 Biotinylated antibody (100x) | 100ul |
| AR1103 | Avidin-Biotin-Peroxidase Complex (100x) | 100ul |
| AR1106-1 | Sample Diluent | 30ml |
| AR1106-2 | Antibody Diluent | 12ml |
| AR1106-3 | Avidin-Biotin-Peroxidase Diluent | 12ml |
| AR1104 | Color Developing Reagent (TMB) | 10ml |
| AR1105 | Stop Solution | 10ml |
| AR1106-5 | Wash Buffer (25x) | 20ml |
| PLA-SEA | Adhesive plate sealers | 4 |
*The kit components are not available for individual purchase.
Materials Required But Not Included In Kit
- Microplate Reader capable of reading absorbance at 450nm.
- Incubator.
- Automated plate washer (optional).
- Pipettes and pipette tips capable of precisely dispensing 0.5 µl through 1 ml volumes of aqueous solutions.
- Multichannel pipettes are recommended for large amount of samples.
- Deionized or distilled water.
- 500ml graduated cylinders.
- Test tubes for dilution.
Data Examples, Quality Control Data & Sample Dilution
Validation Standard Curve O.D. At 450nm
| Concentration (pg/ml) | 0 | 156 | 312 | 625 | 1250 | 2500 | 5000 | 10000 |
| O.D. | 0.009 | 0.060 | 0.107 | 0.195 | 0.357 | 0.681 | 1.255 | 2.011 |
Data Example Images
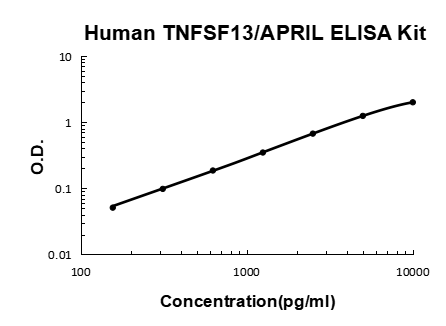
Click image to see more details
Human TNFSF13/APRIL PicoKine ELISA Kit standard curve
Recommended Sample Dilution Ratios
According to our internal validation assays using this ELISA kit, to detect APRIL/TNFSF13, Dilution ratio of 1:1, concentration in serum and plasma is less than the lowest standard, 156 pg/ml..
Some articles we found to cite concentrations of APRIL/TNFSF13 in samples: 17307753 (Pubmed IDs).
Intra Assay Consistency & Inter Assay Consistency
We measured random samples of Human TNFSF13/APRIL ELISA Kit PicoKine® within the same batch/lot to ensure the consistency of the kits' performances. ELISA intra assay consistency is measured using wells from the same plate/assay kit. ELISA inter assay consistency is measured using wells from different plates from the same batch production/lot.
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 16 | 16 | 16 | 24 | 24 | 24 |
| Mean (pg/ml) | 264 | 1700 | 4235 | 241 | 1784 | 4408 |
| Standard deviation | 14.25 | 119 | 237.16 | 15.42 | 144.5 | 317.37 |
| CV (%) | 5.4% | 7% | 5.6% | 6.4% | 8.1% | 7.2% |
Reproducibility
We ensure reproducibility by testing three samples with differing concentrations of APRIL/TNFSF13 in ELISA kits from four different production batches/lots.
| Lots | Lot 1 (pg/ml) | Lot 2 (pg/ml) | Lot 3 (pg/ml) | Lot 4 (pg/ml) | Mean (pg/ml) | Standard Deviation | CV (%) |
|---|---|---|---|---|---|---|---|
| Sample 1 | 264 | 265 | 272 | 285 | 271 | 8.38 | 3% |
| Sample 2 | 1700 | 1548 | 1563 | 1481 | 1573 | 79.55 | 5% |
| Sample 3 | 4235 | 4377 | 4466 | 4871 | 4487 | 236.37 | 5.2% |
Protein Target Info & Infographic
Gene/Protein Information For TNFSF13 (Source: Uniprot.Org, NCBI)
Gene Name
TNFSF13
Full Name
Tumor necrosis factor ligand superfamily member 13
Weight
24279 MW
Superfamily
tumor necrosis factor family
Alternative Names
Tumor necrosis factor (Ligand) superfamily member 13 transcript variant delta ;Tumor necrosis factor ligand superfamily member 13 ;TNFSF13 ; TNFSF13 APRIL, CD256, TALL-2, TALL2, TNLG7B, TRDL-1, UNQ383/PRO715, ZTNF2 TNF superfamily member 13 tumor necrosis factor ligand superfamily member 13|TNF- and APOL-related leukocyte expressed ligand 2|a proliferation-inducing ligand|tumor necrosis factor (ligand) superfamily, member 13|tumor necrosis factor ligand 7B|tumor necrosis factor superfamily member 13|tumor necrosis factor-like protein ZTNF2|tumor necrosis factor-related death ligand-1
*if product is indicated to react with multiple species, protein info is based on the gene entry specified above in "species".For more info on TNFSF13, check out the TNFSF13 Infographic

We have 30,000+ of these available, one for each gene! check them out.
In this infographic you will see the following information for TNFSF13: database IDs, super-family, protein function, synonyms, molecular weight, chromosomal locations, tissues of expression, subcellular locations, post translational modifications, and related diseases, research areas & pathways. If you want to see more information included, or would like to contribute to it and be acknowledged, please contact us [email protected].
Specific Publications For Human TNFSF13/APRIL ELISA Kit PicoKine® (EK0921)
Hello CJ!
EK0921 has been cited in 3 publications:
*The publications in this section are manually curated by our staff scientists. They may differ from Bioz's machine gathered results. Both are accurate. If you find a publication citing this product but is missing from this list, please let us know we will issue you a thank-you coupon.
Prognostic Significance of Serum BAFF, APRIL, TACI and BCMA Levels in Chronic Lymphocytic Leukemia
AB0018 B cell disturbance in rheumatoid arthritis patients: comparative study between treated and non-treated patients
Species: Human
G??zmen S, Karapnar Th, T??fek??i O, Vergin C, Y??ksel F, Irken G, Oren H. Blood Coagul Fibrinolysis. 2014 Jun 6. [Epub Ahead Of Print] B-Cell-Activating Factor, A Proliferation Inducing Ligand And Co-Stimulatory Molecules In The Pathogenesis Of I...
Recommended Resources
Here are featured tools and databases that you might find useful.
- Boster's Pathways Library
- Protein Databases
- Bioscience Research Protocol Resources
- Data Processing & Analysis Software
- Photo Editing Software
- Scientific Literature Resources
- Research Paper Management Tools
- Molecular Biology Software
- Primer Design Tools
- Bioinformatics Tools
- Phylogenetic Tree Analysis
Customer Reviews
Have you used Human TNFSF13/APRIL ELISA Kit PicoKine®?
Submit a review and receive an Amazon gift card.
- $30 for a review with an image
0 Reviews For Human TNFSF13/APRIL ELISA Kit PicoKine®
Customer Q&As
Have a question?
Find answers in Q&As, reviews.
Can't find your answer?
Submit your question
8 Customer Q&As for Human TNFSF13/APRIL ELISA Kit PicoKine®
Question
Q: if the enzyme conjugated APRIL antibodies are mixed with the substrate, will that change the substrate into the enzymatic reaction product? Or the enzyme function is only activated when the antibody is attached with the APRIL antigen?
L. Clark
Verified customer
Asked: 2020-11-22
Answer
A: The enzyme is always active. Avoid contaminating the substrate with enzyme prior to the incubation otherwise it compromises the assay with false positive signal.
Boster Scientific Support
Answered: 2020-11-22
Question
Q: we need your suggestion regarding the dilution ratio of serum samples for detection of APRIL in Human plasma? I am trying to measure a multiple analytes and it requires 100ul of diluted samples for each well. We have limited sample volumes so we like to dilute as much as possible.
S. Chen
Verified customer
Asked: 2020-11-21
Answer
A: having little idea about the physiological or pathological context of your samples we cannot recommend a dilution ratio without performing a pilot test with your samples. Here is how you can perform a pilot study on your own: perform a serial dilution of your samples on the APRIL ELISA kit to make sure you have a linear ascending curve followed by a plateau, which signifies the samples saturating the detection limit of the kit. Then you can pick the dilution ratios from samples in the linear part of the curve as your experimental dilution ratio.
If you are interested in using our ELISA service, you can also send us your sample and we will take care of everything for you. You can check our service details here: bosterbio.com/services/assay-services/ELISA-testing-service
Since you mentioned you have limited samples, our cost effective multiplex ELISA service would fit perfectly for your needs, where we can generate dozens of data points using as little as 25ul sample volume. Information on this service is also in the above link.
Boster Scientific Support
Answered: 2020-11-21
Question
Q: Can APRIL ELISA Kits be used with tissue homogenates (or other non-validated sample types)?
W. Phillips
Verified customer
Asked: 2020-10-05
Answer
A: Unfortunately, Boster Bio has not routinely validated tissue homogenates as a sample type for ELISA kits. This does not mean that ELISA kits are not suitable for other sample types than we have tested: it means further investigation is required. One will need to perform a spike and recovery study to determine if an unvalidated sample type will work with a particular kit. To perform a spike and recovery experiment, one should divide a sample into two aliquots. In one of the aliquots, the user should spike in a known amount of the kit standard. a dilution series is performed comparing the spiked versus the unspiked sample. Generally, samples with expected recovery and linearity between 80-120% are considered acceptable. This method can be used to validate any sample type that has not been evaluated by Boster Bio. for a more detailed spike and recovery protocol, please contact technical support.
Note: acceptable ranges should be determined individually by each laboratory. Additionally, technical support can help determine if a buffer component is not compatible with a given ELISA kit. please view the Citations tab on the product webpage for peer-reviewed papers utilizing a wide range of sample types. We also have an innovator's reward program where if the user validates our ELISA kits in applications or samples previously not validated by Boster Bio or other users, and share such information with us by submit a review, we will reward the user's efforts with a free antibody or ELISA kit from our catalog. Biocompare.com will also give $20 Amazon giftcard as an additional reward, if the review is submitted there as well.
Boster Scientific Support
Answered: 2020-10-05
Question
Q: how are cell lysates prepared for use in Picokine® ELISA kits?
L. Kapoor
Verified customer
Asked: 2019-10-05
Answer
A: in those Picokine® ELISAs where cell or tissue lysate is a validated sample type, sample preparation instructions for lysate are present in the product insert. Components in lysate and lysis buffer can affect immunoreactivity, so if lysate is not a validated sample type, care must be taken in sample preparation and validation.
Boster Scientific Support
Answered: 2019-10-05
Question
Q: is there any online tool I can use to streamline the data analysis for my ELISA results?
M. Sunder
Verified customer
Asked: 2018-10-29
Answer
A: We have a web based ELISA curve fitting (4pl) and data analysis tool. Please give it a try: bosterbio.com/biology-research-tools/ELISA-data-analysis-online. You can also consult our article on ELISA data analysis: bosterbio.com/ELISA-data-analysis-instructions
Boster Scientific Support
Answered: 2018-10-29
Question
Q: is it okay to use citrate plasma as samples in Human APRIL Picokine® ELISA Kit (Catalog # EK0921)?
Verified Customer
Verified customer
Asked: 2018-10-27
Answer
A: Chelating agents such as EDTA, Heparin and Citrate can sequester metal ions from the functional domain of APRIL causing disruption of its protein structure. APRIL may be denatured as a result and may compromise the assay's measurements. The chilating sites could also be too close to the epitopes needed for detection and block the antigen antibody reaction. We have tested the APRIL ELISA, treating samples with a number of anticoagulants and decided that heparin or EDTA can be used for treatment of blood/plasma samples. Do not use other anticoagulents when collecting samples.
Boster Scientific Support
Answered: 2018-10-27
Question
Q: What is the optimal O.D. value for APRIL ELISA kit? I performed your APRIL ELISA on serum samples. For my positive control, I received an O.D. value of 0.826, while my negative control received a value of 0.136. I obtained both of these controls from the ELISA kit, where your kit's typical data shows O.D. values much higher than my positive control and your background is lower. My samples O.D. values are around 0.225 and the highest is only 0.357. is it safe to say these samples contain APRIL even though the O.D. values are not very high?
K. Lee
Verified customer
Asked: 2018-10-07
Answer
A: The absolute O.D. values may change according to incubation time. The more you incubate the higher the O.D. values are going to be. a point of focus should be is whether your sample O.D. values are statistically significantly higher than your blank values. regarding your assay, you could extend your development time in the substrate incubation step to obtain higher O.D. values, as long as your negative controls' O.D. values are not increasing faster in relation to your positive controls. normally, a sample with O.D. value 2 standard deviations higher than your negative controls can be considered positive. We calculate the sensitivity of this ELISA kit by converting cutoff O.D. value, calculated as the average of 20 negative controls plus 2 standard deviations of the 20 negative controls, into a concentration. in other words, when we claim this APRIL ELISA kit to have sensitivity of 10pg/ml, that means the minimum amount of APRIL that can be declared/interpreted as positive by the above standard is 10pg/ml.
Boster Scientific Support
Answered: 2018-10-07
Question
Q: Are Boster Bio recombinant proteins and antibodies sterile?
E. Babu
Verified customer
Asked: 2016-04-03
Answer
A: although the vials are bottled using aseptic techniques, heat-treated vials, and sterile stock solutions, they are not considered or guaranteed to be sterile. If sterile material is required for an experiment, the material can be filtered through a 0.2 micron filter designed for use with biological fluids.
Boster Scientific Support
Answered: 2016-04-03


