This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
Boster Bio Antibody Solutions
Boster Bio offers high yield recombinant antibody production for projects of all scales. Whether you want a partner for complete antibody production or to manage capacity bottlenecks, you can benefit from our expertise.
Starting from antibody sequences, our streamlined platform can yield 1mg antibody for $300/rAb (bulk price, 2 weeks) or scale up to 1g production for $6000 (4 weeks). Contact us today for the most efficient rAb production service.
Begin Inquiry
Recombinant antibodies are monoclonal antibodies produced in vitro from synthetic gene sequneces. Recombinant antibody production is a crucial part of antibody engineering which enables antibody-based therapeutics. Compared to traditional antibody production methods, such as hybridoma, recombinantly produced antibodies are faster, have better consistency, and scalability. Since they are produced in a controled, animal free environment, they can achieve high purity and low endotoxin levels, and can be adopted to the GMP standard.
Recombinant antibodies are often used in cancer therapeutics, treatments of autoimmune disesase, etc. Common frameworks for recombinant engineered antibodies include native, scFv, VHH, and bi/tri specific antibodies.
Service Highlights
Decades of experience and optimized platform maximizes success.
1mg for $600/rAb, bulk price as low as $300/rAb. 1 gram for $6000.
scFv, VHH, chimeric, bi/tri-specific, Fc-fusion, full length etc.
High throughput express 100+ antibody clones, Gram level in 4 weeks.
From sequence to antibody in 2 weeks. Gram level in 4 weeks.
Mitigate project risk by utilizing US-based project support.
Humanization, affinity purification, Isotype and framework switching.
We offer CRO service in WB, IHC, IF, ELISA, FACS, Biocore SPR, etc.
Company stats and facility shots
CiteAb Awards
Fastest Growing
30+ Years
antibody experience
60,000+
publications
Expertise
in rAb expression
40,000+
antibodies made
facility images

Cell Line Construction Lab

Applikon Fermentor

Cell Incubators
1. Submit sequences
2. Get A Quote
3. Place Order
One stop shop for all your antibody needs: discovery, characterization, engineering and production.
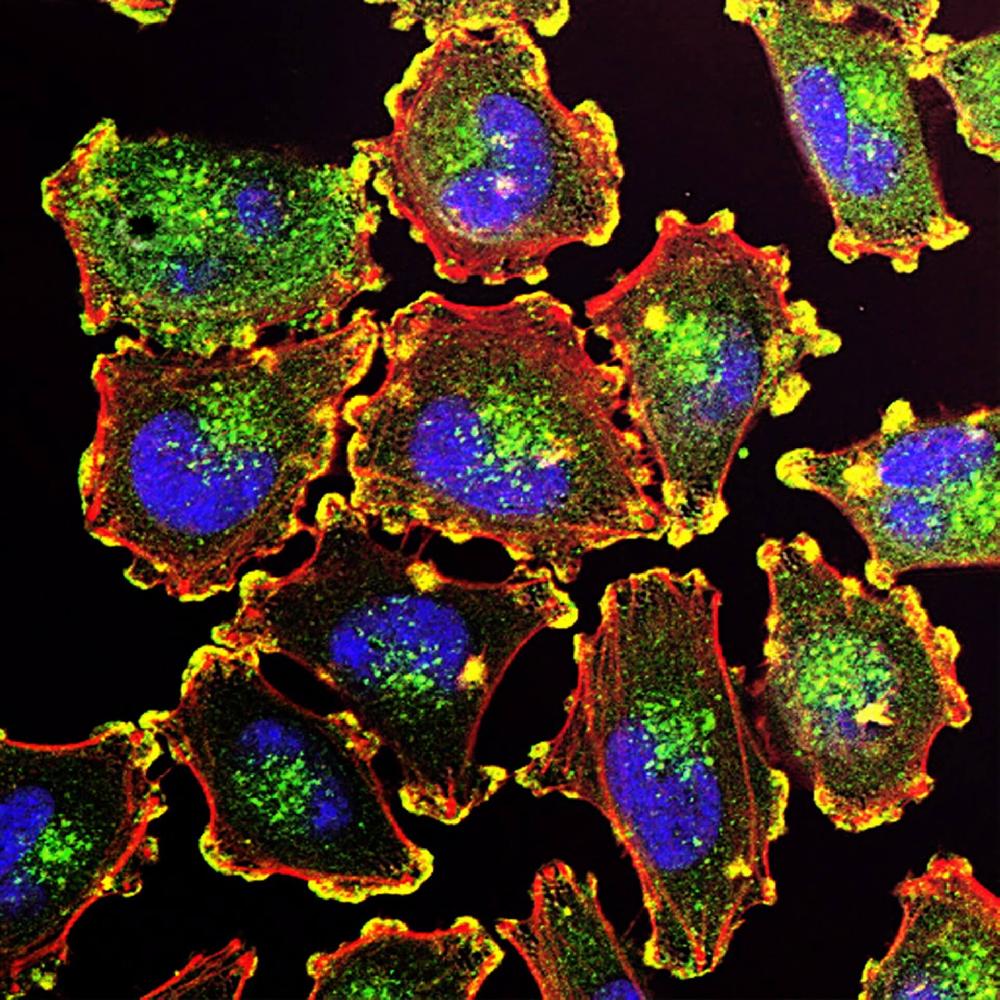
Recombinant Rabbit Monoclonal Antibody Discovery Service
See details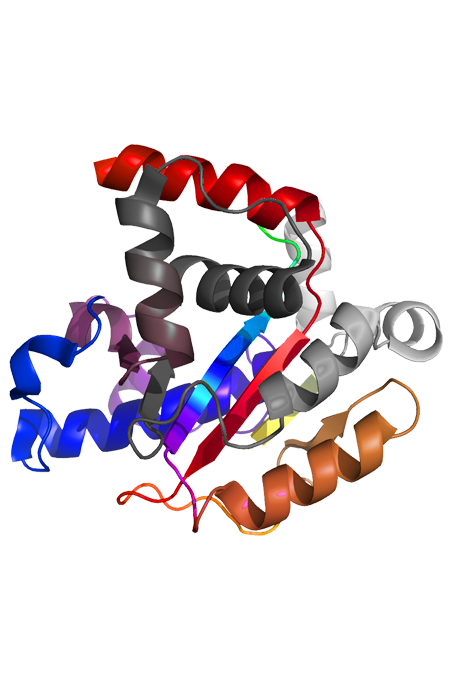
Antibody Conjugation Service
See details
Antibody Pair Development Service
See details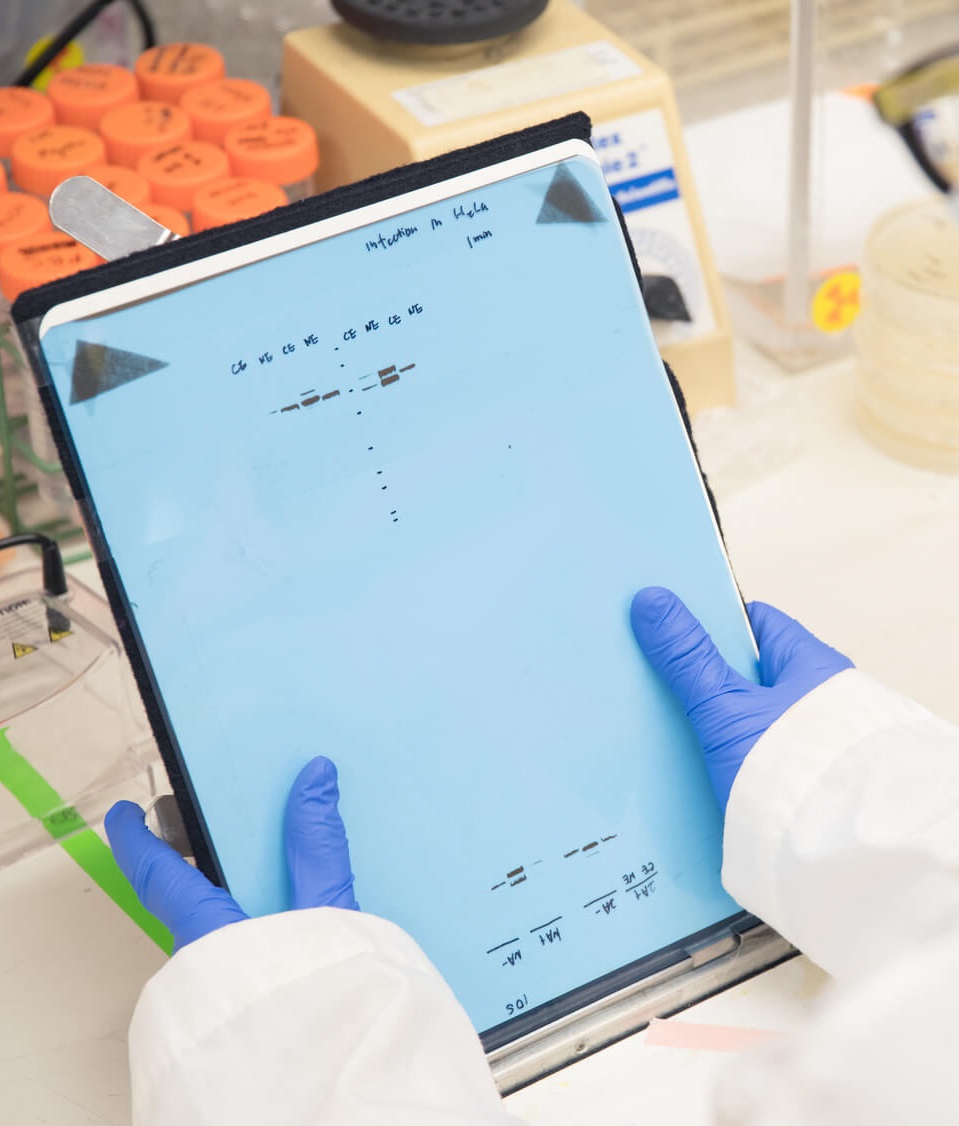
Western Blot Validation Service
See details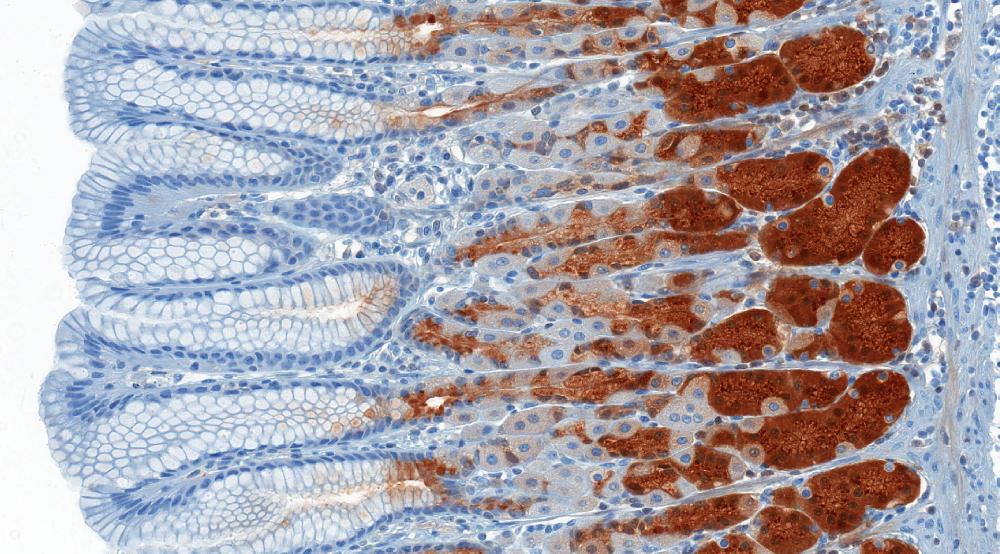
Immunohistochemistry Service
See details
ELISA Services
See detailsSample Report
Get a Quote
Frequently asked questions for recombinant antibody production service.
Q1. What is recombinant antibody production?
Q2. What are the advantages of using recombinant antibodies?
Q3. Which host cells are used for recombinant antibody production?
Q4. Can you produce custom antibodies with specific characteristics?
Q5. What is the typical timeline for recombinant antibody production?
Q6. What is the minimum order quantity for recombinant antibody production?
Q7. What quality control measures are in place for recombinant antibodies?
Q8. Can you provide antibodies for therapeutic applications?
Q9. What type of purification methods do you use?
Q10. Do you offer scale-up production for large quantities of antibodies?
Q11. What is the stability and shelf life of recombinant antibodies?
Q12. What types of antibodies can you produce?
Q13. Can you provide a certificate of analysis (CoA) for the produced antibodies?
Q14. How do I get started with your recombinant antibody production service?