This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
Facts about Cullin-associated NEDD8-dissociated protein 1.

The exchange activity of CAND1 is coupled with cycles of neddylation conjugation: in the deneddylated state, cullin-binding CAND1 binds CUL1-RBX1, increasing dissociation of the SCF complex and promoting exchange of their F-box protein. Probably plays a similar role in other cullin-RING E3 ubiquitin ligase complexes.
| Human | |
|---|---|
| Gene Name: | CAND1 |
| Uniprot: | Q86VP6 |
| Entrez: | 55832 |
| Belongs to: |
|---|
| CAND family |
cullin-associated and neddylation-dissociated 1; Cullin-associated and neddylation-dissociated protein 1; cullin-associated NEDD8-dissociated protein 1; DKFZp434M1414; FLJ90441; KIAA0829FLJ10114; p120 CAND1; TBP interacting protein; TBP-interacting protein 120A; TBP-interacting protein of 120 kDa A; TIP120AFLJ38691; TIP120FLJ10929
Mass (kDA):
136.376 kDA

| Human | |
|---|---|
| Location: | 12q14.3-q15 |
| Sequence: | 12; NC_000012.12 (67269358..67319953) |
Cytoplasm. Nucleus. Predominantly cytoplasmic.
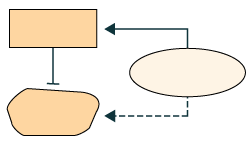

PMID: 12504026 by Zheng J., et al. CAND1 binds to unneddylated CUL1 and regulates the formation of SCF ubiquitin E3 ligase complex.
PMID: 12504025 by Liu J., et al. NEDD8 modification of CUL1 dissociates p120(CAND1), an inhibitor of CUL1-SKP1 binding and SCF ligases.