This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
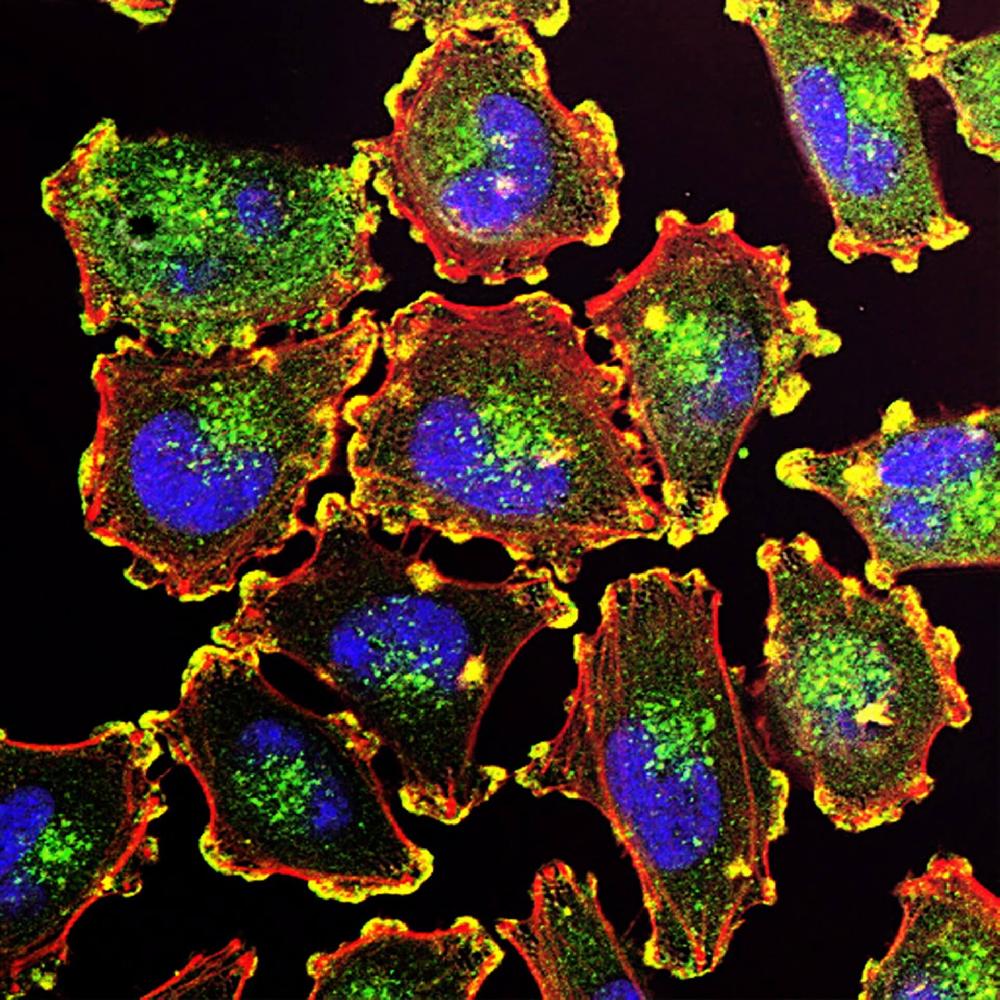
Facts about ATP-dependent zinc metalloprotease YME1L1.

Ensures cell proliferation, keeps normal cristae morphology and complicated I respiration activity, promotes antiapoptotic action and protects mitochondria in the accumulation of oxidatively damaged membrane proteins (PubMed:22262461). Required for normal, constitutive degradation of PRELID1 (PubMed:27495975).
| Human | |
|---|---|
| Gene Name: | YME1L1 |
| Uniprot: | Q96TA2 |
| Entrez: | 10730 |
| Belongs to: |
|---|
| No superfamily |
ATP-dependent metalloprotease FtsH1 homolog; ATP-dependent metalloprotease FtsH1; ATP-dependent metalloprotease YME1L1; EC 3.4.24; EC 3.4.24.-; FTSH; FTSH1; MEG4; Meg-4; PAMPATP-dependent zinc metalloprotease YME1L1; Presenilin-associated metalloprotease; YME1 (S.cerevisiae)-like 1; YME1-like 1 (S. cerevisiae); YME1-like protein 1; YME1Lmeg-4
Mass (kDA):
86.455 kDA

| Human | |
|---|---|
| Location: | 10p12.1 |
| Sequence: | 10; NC_000010.11 (27110111..27155051, complement) |
High expression in cardiac and skeletal muscle mitochondria.
Mitochondrion inner membrane. Mitochondrion.

PMID: 10843804 by Coppola M., et al. Identification and characterization of YME1L1, a novel paraplegin- related gene.
PMID: 18076378 by Guillery O., et al. Metalloprotease-mediated OPA1 processing is modulated by the mitochondrial membrane potential.