This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
The avidin-biotin interaction remains one of the strongest non-covalent interactions between molecules, even surpassing the strength of antibody-antigen interactions. Interactions between these two molecules have been utilized in the laboratory for countless applications, such as immunohistochemistry (IHC), immunoprecipitation (IP), affinity purification, enzyme-linked immunosorbent assay (ELISA), and more. The avidin-biotin-peroxidase complex has been used in countless assays for its simple and effective procedure, incomparable strength of binding between molecules, and ability to label and quantify lowly abundant proteins of interest in experiments. Boster Bio conveniently offers an Avidin-Biotin-Peroxidase Complex for ELISA kit (Catalog# AR1103), complete with necessary reagents, for easy use of avidin and biotin in your experiments. If you would like to learn more about avidin, biotin, and their applications in research, read below and view Boster’s simple protocol.
Avidin is a basic glycoprotein derived from the eggs of birds and reptiles. The stable protein avidin forms a tetramer (four subunits of avidin bind via protein-protein interactions) that is well-known for its ability to bind to the vitamin biotin.
Biotin, also known as vitamin H or B7, is an important nutrient in human health and a small molecule used in research. Due to its nearly irreversible interaction with avidin and avidin analogs, biotin has been conjugated with other proteins for amplified detection of proteins of interest. Antibodies can be biotinylated (coated with biotin molecules) to help detect or label rare proteins without using radioactive techniques.
Since avidin forms a tetramer, avidin can bind to four biotin molecules—one biotin molecule per each avidin subunit. The specificity and stability of an avidin-biotin bond is unparalleled. Because of this, avidin and biotin are commonly utilized for the detection and labeling of proteins. As a testament to the strength of their interaction, avidin-biotin interactions are resistant to enzymatic reactions, changes in pH, denaturing and organic reagents, and temperature changes. It should be noted that avidin does have non-specific binding, which has been improved in non-glycosylated avidin derivatives such as Neutravidin and Streptavidin.
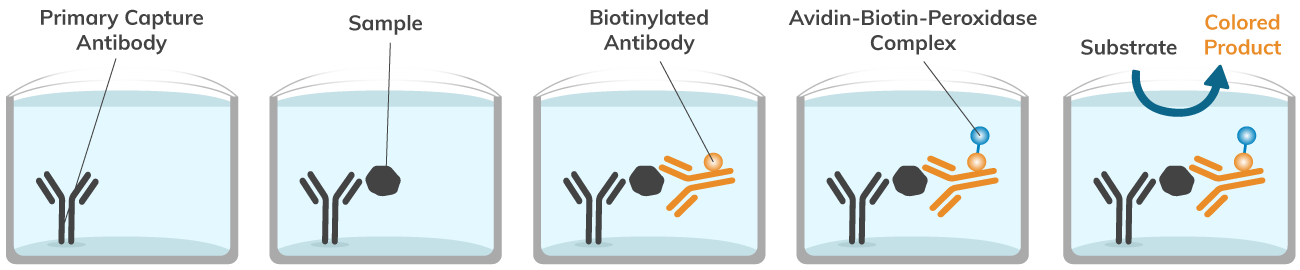
The avidin-biotin-peroxidase-complex, or ABC method, takes advantage of the nearly irreversible interaction between the small molecule biotin and basic glycoprotein avidin. The ABC method can be used in an ELISA to conveniently detect a protein of interest. First, a primary antibody binds to your target protein, then a biotinylated secondary antibody interacts with your protein of interest. Once the primary and secondary antibodies have incubated with your sample, capturing your target protein, the avidin-biotin-peroxidase-complex is added. The avidin-biotin-peroxidase-complex works as follows: avidin binds irreversibly to biotin, while biotin is bound to a peroxidase. The avidin in the avidin-biotin-peroxidase-complex binds to the biotinylated secondary antibodies, forming a multi-unit complex. Finally, a substrate capable of reacting with the peroxidase in the complex is added, such as Horseradish peroxidase (HRP). The reaction between peroxidase and the HRP substrate forms a blue product that can easily be quantified with a spectrophotometer. This simple, in vitro assay can be used to detect and quantify your protein of interest. Boster Bio’s Avidin-Biotin-Peroxidase Complex for ELISA kit (Catalog# AR1103) utilizes the avidin-biotin interaction to help you detect and quantify proteins of interest, even in low abundance, without needing radioactive reagents or complex techniques.
The avidin and biotin interaction can be used in numerous assays, such as ELISA, IHC, IP, and affinity purification. As mentioned above, ELISA assays typically use the ABC method to detect and quantify levels of a target protein in a sample by measuring a blue-colored product formed from peroxidase and HRP substrate. Avidin and biotin can be used in immunohistochemistry (IHC) to label a target protein in a tissue of interest, also by using the ABC method. Streptavidin, an analog of avidin, can also be used in IHC in a similar way to reduce background staining. Due to the strength of interaction between biotin and avidin or streptavidin, these are ideal molecules to use in immunoprecipitation assays for pull-down of proteins of interest. Similarly, biotinylated proteins of interest can be purified with avidin or streptavidin using affinity purification. However, this method does require that your protein of interest be tagged with biotin prior.
While avidin was originally used to bind to biotin, other analogs or derivatives of avidin have been utilized for similar experiments. For example, Neutravidin is a slightly smaller, mildly acidic derivative of avidin which is non-glycosylated. Because it is non-glycosylated and has altered pH, it has much less non-specific binding to cellular proteins. A more commonly used analog of avidin is streptavidin. Streptavidin is produced from the bacterium Streptomyces avidinii and is also a smaller, non-glycosylated, and slightly acidic version of avidin. Both Streptavidin and Neutravidin form tetramers and can bind four biotin molecules.