This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
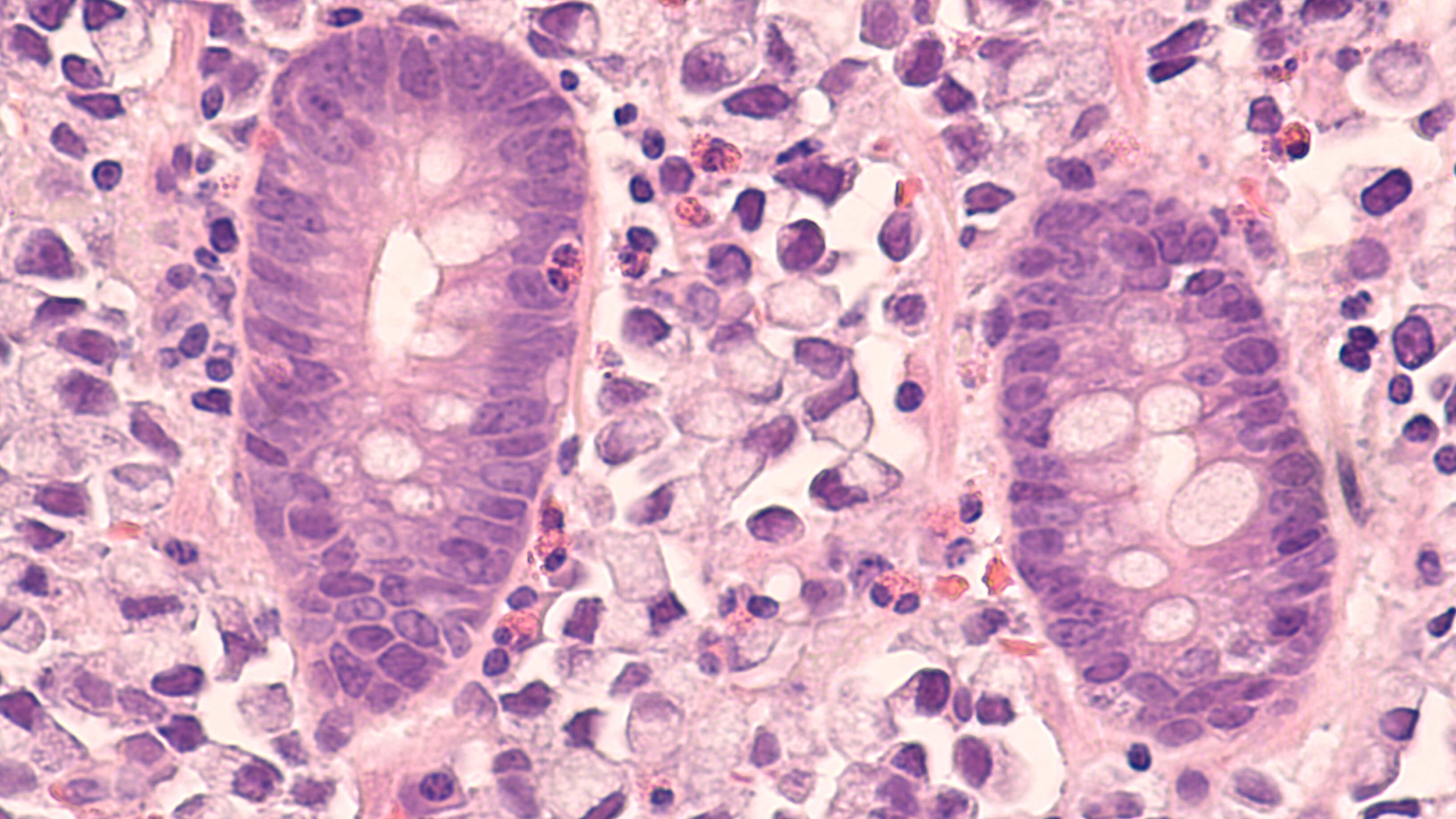
Immunohistochemistry (IHC) is a popular protein detection method that utilizes antibody-antigen interactions to visualize the distribution and localization of specific cellular components within cells and in their proper tissue context.
In the lab, researchers invest time and effort to optimize the sample preparation and sample staining processes of IHC. When successful, the results produce a strong and specific signal.
However, how do we know the results have been interpreted correctly?
When designing your IHC experiment, it is crucial to include positive and negative controls. The controls will help verify whether or not the IHC staining results observed are accurate and valid while exposing experimental artefacts.
Currently, there are 6 established IHC controls that will help you determine the specificity of the observed antibody staining.
For positive tissue controls, a tissue type known to express your protein of interest is stained. If the results are positive, then the assay is working correctly. If no staining occurs in the positive control, it’s time to troubleshoot the staining protocol
To identify suitable positive controls, a good starting place is to check the antibody’s datasheet. Boster Bio provides the Uniprot ID on the datasheet so that you will be able to access a list of tissues that express your protein interest, which can be used as positive controls.
Negative tissue controls are used to reveal non-specific binding and false positive results. Choose a tissue known not to express the protein of interest. Negative controls should not express the target antigen. If staining occurs, you can confirm that the staining is unspecific. Common negative control tissues are knockdown (KD) or knockout (KO) tissue samples.
Certain cells and tissue types, in particular those abundant in collagen, elastin, and lipofuscin, possess inherent biological properties that emit natural fluorescence (aka autofluorescence). If this is unnoticed, it could be misinterpreted as positive staining when it is actually background staining. To prevent this from happening, examine a section of the tissue with a fluorescence microscope for fluorescent labels or a bright-field microscope for chromogenic labels to confirm that the tissue won’t display endogenous background.
No primary controls (aka secondary antibody only controls), as the name suggests, follow the same protocol, except the tissue is not incubated with primary antibodies. The samples are incubated with only the antibody diluent without adding the primary antibody. Afterwards, the samples are incubated with the secondary antibody and other detection reagents. This is to determine if the secondary antibody is binding nonspecifically to cellular components, resulting in false positives or nonspecific binding.
When working with monoclonal antibodies, consider including isotype controls in your experiment. An isotype control is an antibody of the same isotype (e.g. IgG2, IgM, IgY), clonality, conjugate, and host species as the primary antibody, which targets a molecule that is not present in the sample. For example, the target could be a chemical or a non-mammalian protein.
Instead of incubating the sample with the specific primary antibody, the isotype control sample is incubated with the isotype control antibody at the same concentration under the same experimental conditions. The result of this control will help validate that the observed staining is indeed specific and not produced by nonspecific interactions of the antibody with the samples.
Absorption controls are utilized to test if the primary antibody binds specifically to the antigen of interest. The antibody is first incubated overnight with the immunogen. After saturation, the inactivated antibody replaces the primary antibody in the IHC protocol for the control. This should lead to little or no staining of the tissue at the specific antibody binding sites when compared with the observed staining.
It is important to keep in mind that the absorption control will work better if the immunogen is a purified peptide. If the immunogen is a whole protein, nonspecific binding might take place between the protein and the tissue, leading to incorrect interpretations.
Now that you’ve read about 6 types of IHC controls, be sure to include positive and negative controls in your IHC experiment to validate your results!
Access our Technical Resource Center for more IHC tips and troubleshooting guidance.
Unlock new dimensions of biological insight with multiplex IHC! By examining multiple biomarkers simultaneously, our cutting-edge platform allows you to unravel intricate cellular interactions and gain valuable insights for your research.
Questions? Contact [email protected] and we will be happy to assist you.