This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
Representing 5-20% of the circulating group 1 innate lymphoid cells (ILCs), natural killer (NK) cells play a crucial role in the innate immune system. They were named ‘natural killer’ due to their ability to recognize and kill physiologically stressed cells (tumor cells or virus-infected cells) without the presence/activation of typical markers for immune system activation, such as antibodies or major histocompatibility complex (MHC) on cell surfaces (Abel et al., 2018; Guia et al., 2018; Poli et al., 2018).
NK cells are best known for their faster immune reaction (compared to other immune cells) through rapid cytotoxic/cytolytic responses and the production of pro-inflammatory cytokines in response to receptor stimulation—suggesting their promising immunotherapeutic role on detecting and controlling tumor or cancer growth, as well as protection against diseases (Abel et al., 2018; Poli et al., 2018).
The primary sites of NK cell development and maturation are the bone marrow and secondary lymphoid tissues (thymus, lymph nodes, liver, spleen, tonsils, uterus, pancreas). Differentiation and development from common lymphoid progenitor to mature NK cells with specific receptors and distinct effector functions rely on multiple intrinsic (transcription factors) and extrinsic (cytokines and growth factors) signals—such as IL-15, Nfil3, PU.1, Tox, etc (Abel et al., 2018).
NK cells regulate their immunomodulatory responses through an integrated balance of signals derived from their activating and inhibitory receptors. NK cells receptors recognize and interact with different ligands in the form of surface receptors on target cells and stress-associated molecules—such as macrophage-derived cytokines (MHC class I), viral antigens, human leukocyte antigen (HLA-1), surface glycoproteins, dendritic cells through secreted cytokines (IFN-γ, IL-13, TNF-α, TRAIL), chemokines (CCL3, CCL4, CCL5) and growth factor (GM-CSF) (Abel et al., 2018; Yokoyama & Riley, 2008; Poli et al., 2018).
Based on their structures, there are two distinct families of NK cell receptors—the immunoglobulin-like receptors (KIRs) and C-type lectin-like receptors (CD94/NKG2s, Ly49). Among the different types of activating and inhibitory NK cells receptors, Table 1 shows several NK cells receptors and their ligands (Paul & Lal, 2017; Yokoyama & Riley, 2008).
Table 1. Activating and inhibitory receptors of NK cells (Paul & Lal, 2017; Yokoyama & Riley, 2008)
| Type | Receptors | Ligands | |
|---|---|---|---|
| Activating Receptors | NKG2D | MIC-A / MIC-B, ULBP1-4 | |
| CD94/NKG2C | HLA-E | ||
| Ly49H | m157 of MCMV (mouse) | ||
| CD16 | Fc of IgG | ||
| NKp46 | Heparin, influenza hemagglutinin, viral HN | ||
| KIR2DL4 | HLA-G | ||
| KIR2DS1 | HLA-C2 | ||
| KIR2DS2 | HLA-C1 | ||
| KIR2DS4 | HLA-A11 | ||
| KIR3DS1 | HLA-Bw4 | ||
| NKp30 | B7H6, BAT3, pp65 of HCMV, PfEMP1 of Plasmodium falciparum, viral HA | ||
| NKp44 | Viral HA and HN, PCNA, proteoglycans | ||
| DNAM-1 | CD112, CD155 | ||
| Inhibitory Receptors | KIR2DL1 | HLA-C2 | |
| KIR2DL2 | HLA-C1 | ||
| KIR2DL3 | HLA-C1 | ||
| KIR3DL1 | HLA-Bw4 | ||
| KIR3DL2 | HLA-A3, -A11 | ||
| NKR-P1A | LLTI | ||
| CD94/NKG2A | HLA-E | ||
| Ly49A | H-2Dd, Dk, Dp | ||
| Ly49C | |||
| ILT2 (CD85j) | HLA-A, HLA-B, HLA-C, HLA-G1, HCMV UL18 | ||
| CD244 (2B4) | CD48 |
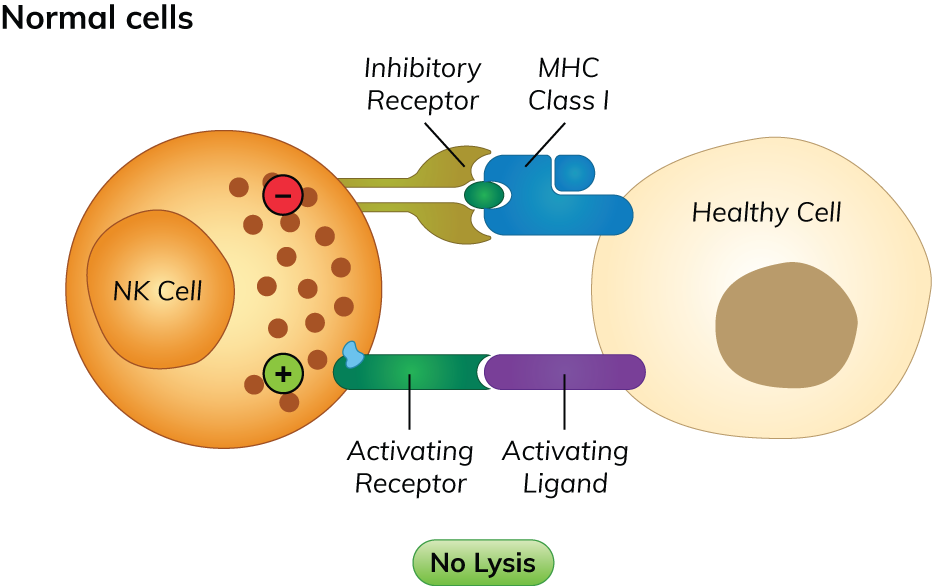
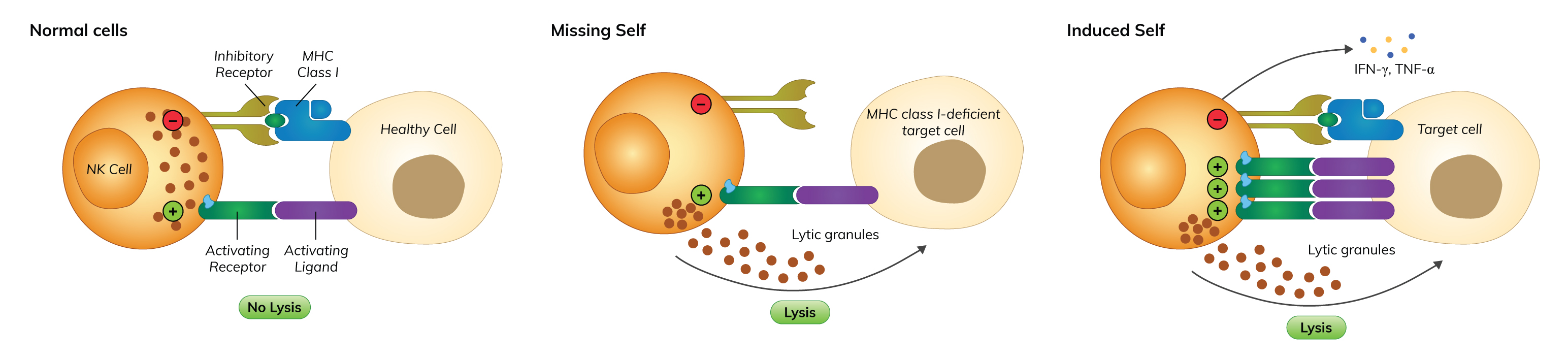
Figure 1. Mechanism of ‘immunological-self’ recognition
NK cells can distinguish between normal cells with stressed cells through their surface molecules. Normal, healthy cells express MHC class I molecules, which are ligands for inhibitory NK cells receptors (human KIRs; Ly49, found in non-human species) on their surfaces. Activation of inhibitory NK cell receptors in response to surface MHC class I leads to inhibition of NK cell activation (Abel et al., 2018; Poli et al., 2018).
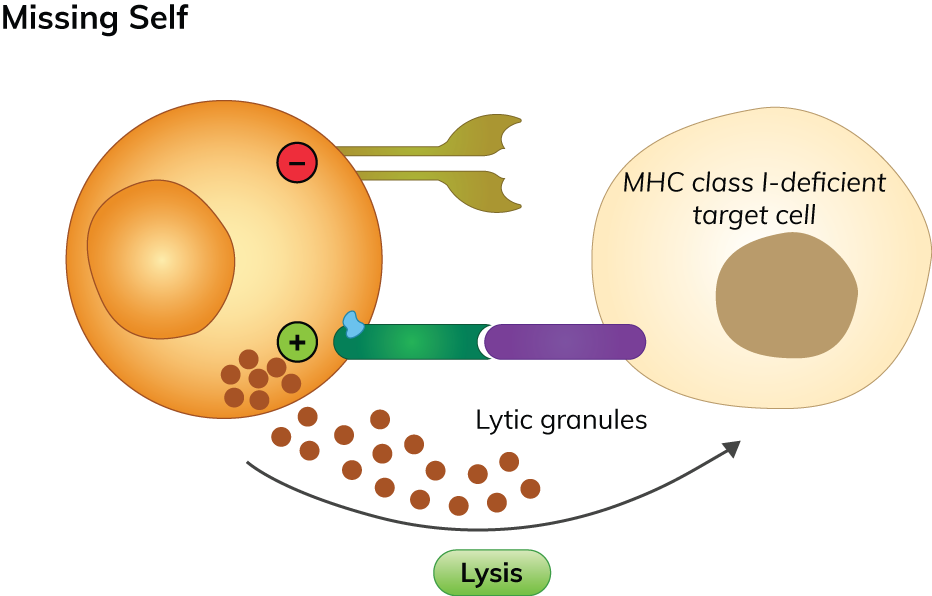
Figure 2. Mechanism of ‘missing-self’ recognition
Stressed cells (tumor cells or virus-infected cells) transform and lose their MHC class I molecules. NK cells are also able to detect the lack or downregulation of these ligands in stressed cells—identifying them as target cells and trigger cell-mediated cytolytic activity through the release of cytotoxic granules containing granzymes (perforin and protease) from their cytoplasm to induce apoptosis or osmotic cell lysis. In addition to this, NK cells also secrete various antimicrobial molecules and α-defensins—causing cell wall disruption and directly kill the cells. Simultaneously, the lack of inhibitory receptor ligands will also result in lower inhibition of NK cells and induce NK cell activation (Abel et al., 2018; Poli et al., 2018).

Figure 3. Mechanism of ‘induced-self’ recognition
Stressed cells also often overexpress and secrete various cytokines that are ligands for activating NK cell receptors—such as IL-12, IL-2, IL-15, IL-18, CCL5, etc. Increased circulating cytokines within the affected site will increase the expression of activating NK cells receptors—leading to secretion of the key pro-inflammatory cytokines IFN-γ and TNF-α to activate various signalling pathways (MAPK, NF‐κB, JAK/STAT) to generate various immune response and promote cell death. With the expression of activating receptors exceeding the loss of inhibitory signalling, NK cell-mediated cytolytic/cytotoxicity activity and secretion of pro-inflammatory cytokines to eliminate target cells are activated, without harming healthy cells (Chan et al., 2014; Abel et al., 2018).
The measurement of different NK cells activity upon stimulation by receptor binding with their selective ligands is a useful biomarker for evaluating changes in functional immune response in the biological fight against cancer, intracellular pathogens and various immune-mediated diseases. Enzyme-linked immunosorbent assay (ELISA) is a widely used method for screening and quantification of NK cells concentration, their cytotoxic capacity and cytokine production in various samples (body fluids, secretions, tissue homogenates, cell culture supernatant, etc.) by measuring the concentrations of several effector molecules produced by NK cells during immune response—such as IFN-γ, TNF-α, IL-12, IL-18, MIP-1β, etc. (Barrow et al., 2018; Abel et al., 2018; Yosef et al., 2015; David et al., 2009).
Among the list of receptors presented in Table 1, the expression and activation of several NK cell receptors (as described below) have become increasingly important markers for the determination of NK cell activity.
The measurement and detection of NK cells activity through ELISA and antibodies is a useful tool for evaluating changes in functional immune response against cancer, intracellular pathogens, and various immune-mediated diseases. Receptors such as NCR2, NCR1, and KIR3DL2 are increasingly important markers for determining NK cell activity. NCR2 and NCR1 receptors recognize cellular ligands expressed on tumor cells, dendritic cells, and viral-infected cells, while the KIR3DL2 inhibitory receptor recognizes human leukocyte antigen class 1 molecules. Using Boster’s antibodies and ELISA kits can facilitate the screening and quantification of NK cells concentration, cytotoxic capacity, and cytokine production in various samples, aiding in the development of more effective treatments for immune-related diseases.
References