This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
Proteins are the workhorse molecules that drive virtually every biological system. With the increasing recognition of the role of proteins in various research and manufacturing activities, simply isolating them from their natural host cells cannot meet the escalating demand of the market. Chemical synthesis is also not a viable option for this endeavor due to the size and complexity of proteins. Instead, the advances made possible by recombinant DNA technology have profound implications in increasing the supply of proteins needed by the rising demand.
Today, living cells and their cellular machinery are usually harnessed as factories for producing the protein of interest in large quantities. Proteins produced with recombinant DNA technology are the so-called recombinant proteins, and every researcher that embarks on a new project that will need a purified protein immediately thinks of how to obtain it in a recombinant form.
To help you get a better understanding of how recombinant proteins are produced, this article will cover these topics:
Recombinant protein production is a mature biotechnological process for large-scale production of specific proteins of interest. In general, recombinant protein production is achieved through the manipulation of gene expression in an organism by fusing sequences of foreign DNA into a host cell. Because all living things share the same DNA structure, the altered DNA can be re-inserted into the host genome, after which it can be replicated, transcribed, and translated to a recombinant protein and further purified. In other words, the steps to obtain recombinant proteins include the expression and purification processes.
Overall, recombinant protein expression involves transfecting host cells with a DNA expression vector containing the gene encoding the recombinant protein of interest and then culturing the cells so that they express the desired protein. The first step in recombinant protein expression is the identification and isolation of the correct gene of interest from the donor cells using restriction enzymes. Amplification of the DNA fragment can be conducted through various methods, such as PCR, ligase chain reaction, and transcription-mediated amplification.
The following step includes the sub-cloning of the recombinant DNA into an expression vector, usually a plasmid or virus. In many cases, the vector is designed to contain additional sequences (strong promoter, translation initiation signals, regulatory elements, resistance markers, etc.) to enhance the expression of the recombinant DNA and aid in the selection process based on specific requirements needed. After, the vector is transformed into the host of choice and replicated through natural DNA-replication processes and cell division.
The selection of a suitable expression system and/or host organism depends on the desired production scale, time/resources available, and the intended use of the recombinant protein, whereas the choice of vector is largely governed by the host. Many host expression systems are available, each with its advantages and disadvantages, as further discussed below.
Before a specific protein can be identified and utilized, the protein must first be separated and purified. The purification process typically includes four phases: (1) protein extraction, (2) precipitation and differential solubilization, (3) ultracentrifugation, and (4) chromatographic methods.
Protein extraction can be accomplished using any number of mechanical, chemical, physical and biological cell lysis techniques. However, to maintain the protein’s native conformation, activity, and integrity, mild detergent-based lysis buffers (Triton X-100, CHAPS, etc.) are more commonly used. Fractional precipitation and/or two-phase partition systems are usually employed for the second step.
The first proteins to be purified are water-soluble. Purification of membrane-bound proteins requires disruption of the cell membrane to isolate any one particular protein that is in the same membrane compartment. Centrifugation is traditionally required to precipitate unwanted cell debris and for clarified lysate recovery.
The target recombinant proteins are then separated from a complex mixture of proteins based on differences in their specific physical, chemical, and biological properties using chromatographic and/or magnetic bead separation methods. There are a wide variety of methods that can be optimized/combined to generate a suitable purification scheme with respect to recovery, resolution, speed and capacity. The chosen purification resin based on the characteristics of the target protein, the vector/host system applied, and the expression site (intracellular/extracellular) are keys to achieving a better chromatographic purification process.
More frequently, two, three, or more purification steps are required to achieve the desired level of purity. Each step will cause some product loss, a yield of 80% in each step is assumed. Thus, it is advisable to have as few purification steps as possible. The most general method to evaluate/monitor the purification process is by running an SDS-PAGE or calculating the absorbance of the proteins (280 nm) through each step. This method only gives a rough measure of the amounts of different proteins in the mixture, and it is not able to distinguish between proteins with similar MW. Therefore, in order to evaluate the process of multistep purification, the specific protein amounts have to be compared to the amount of total protein.
Because purification of native proteins can be challenging, most recombinant proteins are expressed as fusion proteins with short affinity tags. Protein tags are a useful and convenient tool for improving the solubility of recombinant proteins, streamlining protein purification, and enabling an easy way to track proteins during protein expression/purification. Using protein tags also allows for affinity chromatography, which is often considered as one of the most efficient chromatography techniques.
Over the last 30 years, there have been considerable advances in both cell-based and cell-free technologies for expressing recombinant proteins. Cell-based systems can be further classified into eukaryotic and prokaryotic systems. Although the prokaryotic system can express eukaryotic genes, the genes must first be adopted by cloning the cDNA version since the expression system in prokaryotes is different from those of eukaryotes.
Mammalian systems have a higher level of native folding, post-translational modifications, and functional activity than other systems because they represent a physiologically relevant environment. Recombinant protein production in mammalian cells can be conducted transiently or through stable cell lines. While stable cell lines can be used reproducibly over several experiments, transient production offers higher-yield protein production. However, the benefits of these systems are coupled with more demanding cultural conditions. Currently, Chinese hamster ovary (CHO) cells and HEK293 cells are the most widely used mammalian host cells for expressing recombinant proteins.
In this system, insect cells are cotransfected with recombinant baculovirus containing super-strong promoters that are inactive in mammalian cells. The post-translational processing of the proteins expressed using this system closely resembles those expressed in mammalian cells. Therefore, they are more likely to have biological/immunological activities comparable to those that naturally exist in mammalian cells. The insect expression system is particularly well-suited for producing large quantities of recombinant proteins that are difficult to express in bacteria due to size, complexity, or post-translational processing requirements.
Recombinant protein production in yeast is an efficient strategy for producing large amounts of recombinant proteins with high levels of purity. They can be grown to very high cell mass densities so the protein is over-expressed and secreted from the cell for recovery in the culture medium. The proteins expressed have a certain degree of the post-translational processing capacity of mammalian proteins and are more stable than prokaryotic systems. However, due to differences in post-translational glycosylation patterns, this system can only generate eukaryotic proteins that do not require glycosylation. Several yeast species can be used for recombinant protein expression, including Saccharomyces, Schizosaccharomyces pombe, Pichia pastoris and Hansanuela polymorpha.
Bacterial expression systems for recombinant protein production are regarded as the most commonly used, economical, and classical expression systems because of their simple operation, clear genetic background, high expression level, and short culture period. E. coli remains the dominant species for the bacterial production of recombinant proteins. However, eukaryotic proteins expressed in bacteria are often non-functional because the cells are not equipped to accomplish the required post-translational modifications. Also, many proteins become insoluble as inclusion bodies that are very difficult to recover without harsh denaturants and subsequent protein-refolding procedures for recovery.
In recent years, recombinant protein production in plants using microalgae has emerged as a promising platform for large-scale, rapid, and cost-effective recombinant protein production. Microalgae are easily cultivated with basic nutrient requirements and offer expression in both nuclear and chloroplast genomes with some degree of post-translational modifications. However, as a production platform, the technology is still in its infancy and there are still many hurdles to overcome before it can emerge as a mature expression system.
In cell-free systems, synthesis of the protein is carried out in vitro using translation-compatible extracts of whole cells that contain all the components needed for transcription, translation, and even post-translational modification. With additional supplements of cofactors, proteins of interest can even be formed in a matter of hours.
Choosing the right expression system is the key to success, as the host cells will provide the protein synthesis machinery that initiates the outline of the whole process. The following table compares the six protein expression systems, their respective advantages and disadvantages, and the most common applications of each expression strategy.
| Expression System | Advantages | Disadvantages | Most Common Application |
|---|---|---|---|
| Mammalian cells |
|
|
|
| Insect - Baculovirus |
|
|
|
| Yeast |
|
|
|
| Bacterial |
|
|
|
| Algal |
|
|
|
| Cell-free |
|
|
|
Currently, recombinant protein production is one of the most powerful techniques used in a broad range of applications from basic research to pharmaceutical development. There are several advantages using recombinant proteins in various applications over native proteins, including more flexibility and cheaper production costs. However, it should be noted that the recombinant proteins produced in cells may not be the same as the natural forms. This difference may affect the results of experiments and/or reduce the effectiveness of therapeutic recombinant proteins.
Some application examples of recombinant protein production in different research fields are outlined in the table below.
| Applications | Description |
|---|---|
| In vitro and in vivo experiments |
|
| Functional assays |
|
| Standards |
|
| Controls |
|
| Improved or altered protein functions |
|
| Antibody production and profiling |
|
Overall, there are seven basic steps of recombinant protein production:
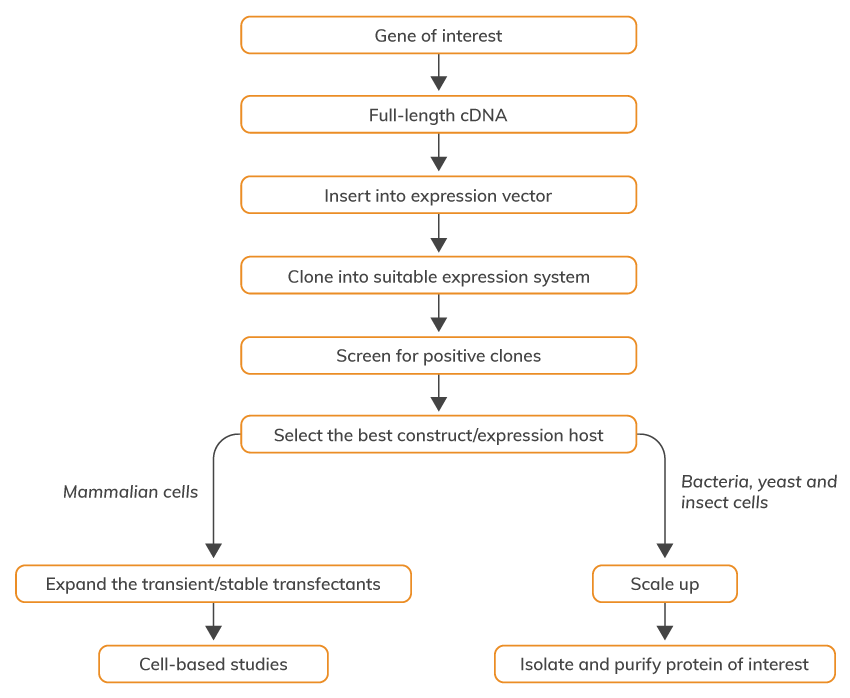
However, even when hundreds of proteins are produced at a commercial scale, the production of recombinant proteins still constitutes a challenge in many cases. There is no universal protocol that can be used and there are several common problems that are often encountered during recombinant protein production, such as the loss of expression, instability of the vector, formation of inclusion bodies, heterologous gene expression, etc. Higher production efficiencies and, consequently, lower costs of the final product are also needed for obtaining a commercially viable process.
Nowadays, increasing evidence has demonstrated that the best results are obtained when both molecular biology and bioengineering approaches are used. Some common strategies for improving recombinant protein production involve the manipulation of the culture conditions using different types of induction strategies, growth control supplementation, and optimized bioreactor and operation parameters.
The production of recombinant proteins is complex, expensive, and time-consuming — especially for most of those who do not have enough experience to express and isolate a recombinant protein. Many biotechnology companies provide custom recombinant protein production services on different scales such as Boster Bio. Visit our recombinant protein expression service page for details and feel free to leave us a message if you have any questions!