Flow cytometry and Fluorescence-Activated Cell Sorting (FACS) are indispensable tools in biomedical research and clinical diagnostics. Despite their widespread use, confusion often arises regarding their terminology and functionalities. In this article, we identify distinctions between flow cytometry and FACS, and discuss their principles and applications.
What is Flow Cytometry?
Developed in the 1950s and 1960s, flow cytometry revolutionized cell analysis by allowing rapid, high-throughput measurement of multiple cellular characteristics. This technique analyzes the physical and chemical characteristics of particles or cells in a fluid suspension, and involves passing a cell-containing fluid stream through a laser beam, measuring the scattered and fluorescent light emitted by the cells.
Key aspects of flow cytometry include:
- Principle: Flow cytometry utilizes lasers to analyze the physical and chemical properties of cells in a fluidic suspension by measuring scattered and emitted fluorescent light, providing multiparametric data on individual cells.
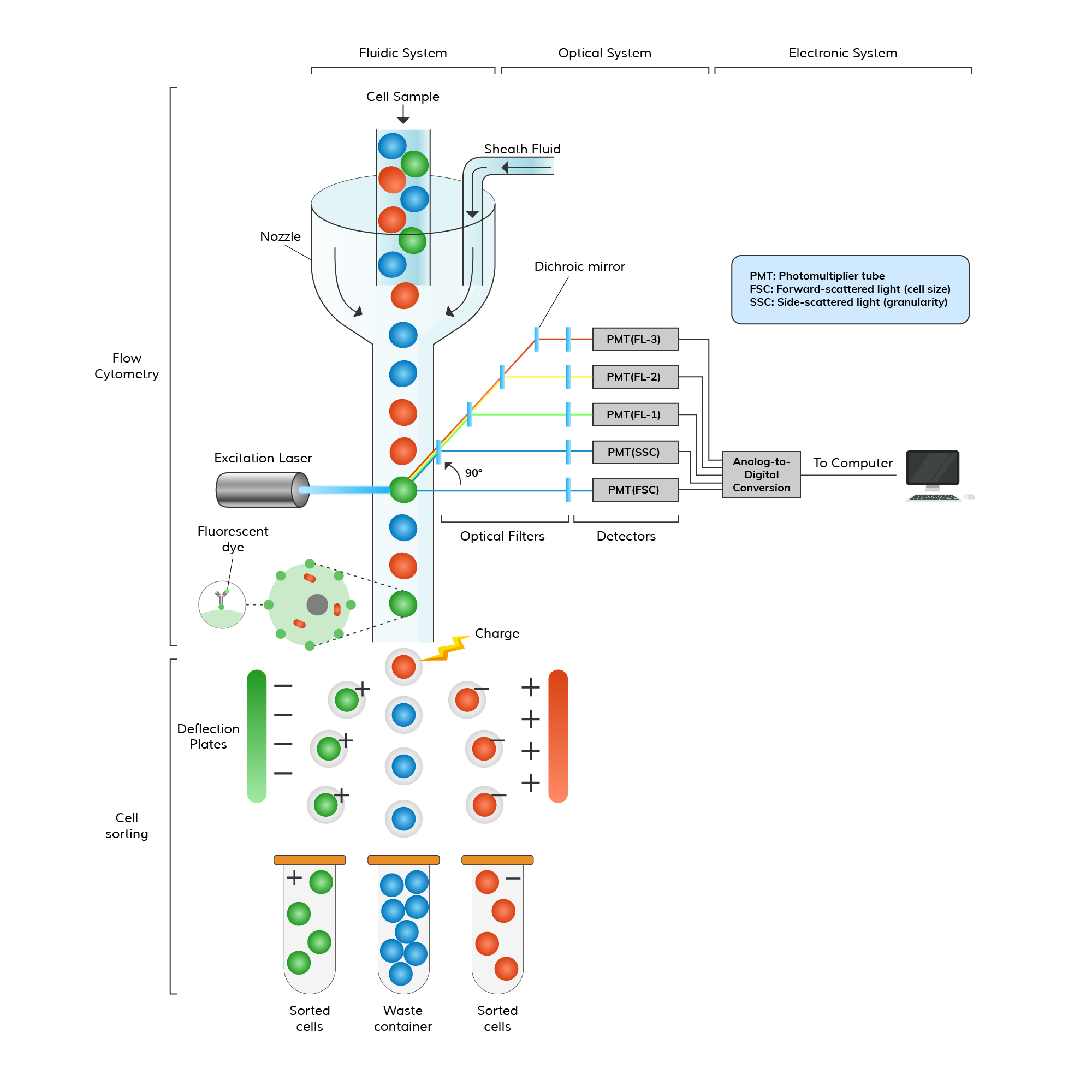
- Components: A typical flow cytometer comprises a fluidics system for sample flow control, lasers for excitation, optical detectors for light and fluorescence measurement, signal processing electronics for data handling, and software for instrument control and data analysis.
- Applications: Widely utilized in immunology, oncology, hematology, and microbiology for cell counting, biomarker detection, and cell sorting.
- Advantages: High-throughput analysis, multiparametric data acquisition, and versatility.
- Limitations: Limited sorting capabilities and complexity in analyzing rare cell populations.
What is FACS?
FACS (Fluorescence-Activated Cell Sorting) emerged in the 1960s as an extension of flow cytometry, introducing the capability to isolate and sort specific cells based on their fluorescent properties, pioneered by Len Herzenberg and colleagues at Stanford University.
Key features of FACS include:
- Principle: FACS utilizes fluorescently-labeled antibodies or dyes to specifically target cells of interest, allowing for their isolation or enrichment.
- Components: In addition to standard flow cytometry components, FACS instruments consist of specialized sorting mechanisms such as charged plates and electrostatic deflection systems, along with more sophisticated software for instrument control and data analysis. These enable precise isolation of specific cell populations based on their fluorescence properties.
- Applications: Essential in cell biology, immunology, and stem cell research for isolating pure cell populations, studying gene expression, and analyzing cell signaling pathways.
- Advantages: Enables the isolation of rare cell subsets, clonal expansion, and downstream molecular analyses.
- Limitations: Higher complexity and cost compared to standard flow cytometry, requiring specialized training and resources for operation and maintenance.
Principle of Flow Cytometry and FACS
In the following diagram, we have illustrated the simplified principle of flow cytometry and FACS.

Key Differences Between Flow Cytometry and FACS
| Aspect | Flow Cytometry | FACS |
|---|
| Sorting Capability | Limited or absent | Ability to sort cells based on fluorescence |
| Instrumentation | Typically involves one laser and detectors | Requires additional sorting components |
| Complexity | Relatively simpler in design and operation | More complex due to sorting mechanisms and specialized training |
| Application Focus | Primarily used for cell analysis | Designed for both analysis and sorting |
The table above summarizes some of the key differences between flow cytometry and FACS. Below, we elaborate on the compared aspects between flow cytometry and FACS.
Sorting Capability
- Flow Cytometry: While flow cytometry can analyze and characterize cells based on their physical and chemical properties, its sorting capability is often limited. It can sort cells based on general characteristics such as size, volume, granularity, and fluorescence intensity, but may struggle with precise sorting of specific cell populations.
- FACS: Fluorescence-Activated Cell Sorting excels in sorting cells with high precision and efficiency based on their fluorescence properties. FACS instruments incorporate specialized sorting mechanisms, such as charged plates and electrostatic deflection systems, enabling the isolation of highly purified cell populations even among complex mixtures.
Instrumentation
- Flow Cytometry: Typically, a flow cytometer involves a single laser and detectors to measure scattered and fluorescent light emitted by cells passing through a fluidic system. It's characterized by its simplicity in design and operation.
- FACS: FACS instruments require additional components beyond standard flow cytometers, including sorting mechanisms (e.g. electrode/charged plates, electrostatic deflection systems) for isolating cells based on their fluorescence properties. This additional complexity is necessary to facilitate the sorting process.
Complexity
- Flow Cytometry: Flow cytometry is generally simpler in design and operation compared to FACS. It primarily focuses on cell analysis, making it more straightforward for routine experimental use.
- FACS: Due to its sorting capabilities, FACS instruments are inherently more complex. They involve intricate sorting mechanisms and require specialized training for operation and data interpretation, adding a layer of complexity to experimental workflows.
Application Focus
- Flow Cytometry: Flow cytometry is predominantly used for cell analysis, including cell counting, biomarker detection, and characterization of cellular properties. It's widely employed across various fields such as immunology, oncology, hematology, and microbiology.
- FACS: While FACS shares many applications with flow cytometry, its unique ability to sort cells based on fluorescence enables additional functionalities. FACS is not only used for cell analysis but also for sorting specific cell populations for downstream applications such as clonal expansion, gene expression analysis, and functional studies.
Common Misconceptions
Common misconceptions of flow cytometry and FACS include:
- Interchangeable Use of Terms: While flow cytometry and FACS are often used interchangeably, they represent distinct techniques with varying capabilities.
- Technical Expertise: Operating a flow cytometer requires a certain level of technical proficiency, but mastering FACS demands specialized training in sorting techniques and data analysis.
Conclusion
Although flow cytometry and FACS share common principles, they exhibit varying capabilities and applications. Flow cytometry offers high-throughput analysis and multiparametric data acquisition while FACS distinguishes itself with its ability to precisely sort cells based on fluorescence properties. The two techniques differ in sorting capability, instrumentation complexity, and application focus. Whether you are a seasoned cytometry expert or a novice researcher, grasping these nuances will effectively aid in optimizing experimental design and data interpretation, harnessing the potential of these powerful analytical tools.
If you’d like to learn more about flow cytometry and FACS, check out Boster’s Flow Cytometry/FACS eBook, which discusses the principle, protocol, and troubleshooting tips for flow cytometry/FACS. Furthermore, our catalog offers 7,000+ flow-validated antibodies for your research.
Feel free to reach out to [email protected] if you have any questions or comments!