This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
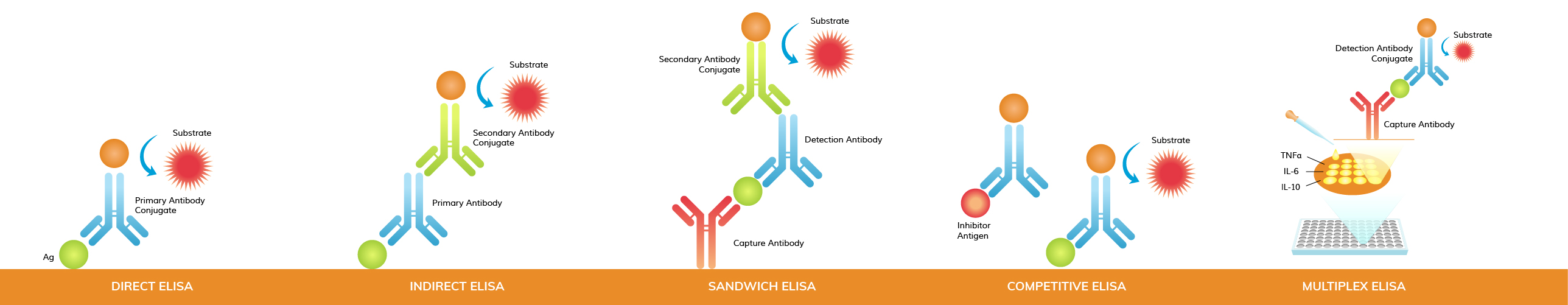
Enzyme-Linked Immunosorbent Assay (ELISA) is a versatile and widely used biochemical technique for detecting and quantifying specific molecules such as proteins, peptides, antibodies, and small molecules. Different types of ELISA—Direct, Indirect, Sandwich, Competitive, and Multiplex—offer distinct methodologies tailored to various research, diagnostic, and clinical applications.
In our blog, we discuss each type of ELISA as well as their unique advantages, considerations, and applications.
ELISA can be categorized into several types based on the method of detection and purpose.

In direct ELISA, the antigen is directly immobilized onto the surface of the microtiter plate. A labeled enzyme-linked antibody specific to the antigen is then added, which binds directly to the antigen. This type of ELISA is relatively simple and quick but may have lower sensitivity compared to other methods.
Advantages:
Disadvantages:
Applications: Direct ELISA can be used for screening antigens in biological samples, quantifying antigens that can directly bind to antibodies without interference, and rapid diagnostic tests due to its simplicity and quick turnaround time.

For indirect ELISA, the antigen is pre-coated similarly to direct ELISA. However, in this method, an unlabeled primary antibody specific to the antigen is added first. Then, a labeled secondary antibody that binds to the primary antibody is introduced. This amplifies the signal, increasing sensitivity. Indirect ELISA is versatile and widely used for detecting antibodies in serum.
Advantages:
Disadvantages:
Applications: Indirect ELISA can detect and quantify antibodies in serum or other biological fluids, screen large numbers of samples for antibody responses (e.g., in serological surveys), and determine antibody titers in vaccine development and immune response studies.

Sandwich ELISA involves immobilizing a capture antibody specific to the antigen of interest onto the microtiter plate. The antigen-containing sample is added, and if the antigen is present, it binds to the capture antibody. Then, a detection antibody that is specific to a different epitope of the antigen is added, forming a "sandwich" with the antigen. A labeled enzyme-linked secondary antibody is then added, which binds to the detection antibody, enabling detection. This method is highly sensitive and specific, making it a suitable and popular choice for detecting antigens in complex samples.
Advantages:
Disadvantages:
Applications: Examples of applications for sandwich ELISA include quantifying specific antigens in complex biological samples (e.g., serum, tissue lysates), detecting biomarkers in disease diagnostics (e.g., detecting tumor markers, cytokines), and monitoring protein expression levels in research and clinical settings.
Join other scientists and browse Boster’s catalog of 2,000+ ELISA kits to find the ELISA kit you need for your research! Our ELISA kits have been extensively validated for sensitivity and reproducibility.

In competitive ELISA, also called inhibition ELISA, the reference antigen is pre-coated on the microtiter plate. The sample containing an unknown amount of antigen is incubated with a known amount of labeled antibody (conjugated to an enzyme), and then added to the wells. The reference antigen and the sample antigen compete for binding to the limited quantity of labeled antibodies. The amount of labeled antibody bound to the plate is inversely proportional to the concentration of antigen in the sample. In other words, the more antigen in the sample, the less reference antigen will be detected and the weaker the output signal.
For some competitive ELISA kits, antibodies, instead of antigens, are pre-coated onto the plate. Afterwards, a known amount of labeled antigen and an unknown amount of sample antigen compete for binding to the antibodies coated on the plate.
Although inhibition ELISA can be more complex due to its competitive nature, inhibition ELISA can be flexibly adapted to use principles from direct, indirect, and sandwich ELISA. The competitive ELISA is useful for quantifying small molecules or detecting inhibitors.
Advantages:
Disadvantages:
Applications: Some applications of competitive ELISA include detecting and quantifying small molecules or inhibitors (e.g., drugs, hormones), assessing antibody neutralization in virology and vaccine research, and screening for contaminants or residues in food, environmental samples, or pharmaceutical products.

Multiplex ELISA allows for the simultaneous detection and quantification of multiple analytes (antigens or antibodies) within a single sample. It is particularly useful in high-throughput screening and diagnostics where multiple biomarkers need to be analyzed simultaneously.
Advantages:
Disadvantages:
Applications: Multiplex ELISA is used for simultaneous detection and quantification of multiple analytes (e.g., cytokines, biomarkers) in a single sample, high-throughput screening in drug discovery and development, and biomarker profiling for disease diagnosis, prognosis, and monitoring. If you’re looking to test your samples with multiplex ELISA assays, check out Boster’s Multiplex Assay Service to design your research experiment.
Below, we’ve summarized each type of ELISA in a table, along with their respective advantages, disadvantages, and typical applications.
| ELISA Type | Description | Advantages | Disadvantages | Typical Applications |
|---|---|---|---|---|
| Direct ELISA | Antigen directly immobilized on plate; labeled antibody binds directly to antigen. | Simple procedure, fewer steps, lower background noise. | Less sensitive, limited to antigens binding directly to antibodies. | Screening for antigen presence, rapid diagnostic tests. |
| Indirect ELISA | Antigen immobilized; primary antibody binds antigen, secondary antibody (labeled) binds primary. | Amplifies signal, higher sensitivity, detects a wide range of antigens. | More complex, increased risk of nonspecific binding. | Antibody detection, quantification in serum, antibody titration. |
| Sandwich ELISA | Capture antibody pre-coated; antigen binds, detection antibody (labeled) binds to antigen "sandwich". | Highly specific and sensitive, less nonspecific binding. | Requires two specific antibodies, technically demanding and costly. | Biomarker detection, quantification of antigens in complex samples. |
| Competitive ELISA | Antigen immobilized; labeled antigen competes with sample antigen for limited antibody binding sites. | Quantifies small molecules, direct measurement of analyte concentration, flexible. | Less sensitive, requires careful optimization of conditions. | Detection of inhibitors, quantification of small molecules. |
| Multiplex ELISA | Simultaneous detection of multiple analytes using labeled antibodies specific to each analyte. | Simultaneous detection, reduced sample volume and labor. | Requires specialized equipment and reagents, complex data analysis and interpretation. | Biomarker profiling, high-throughput screening in drug discovery, simultaneous detection of multiple analytes. |
The range of ELISA techniques—Direct, Indirect, Sandwich, Competitive, and Multiplex—provides researchers and clinicians with powerful tools for precise detection and quantification of biomolecules across various fields. Each ELISA type offers unique advantages, such as simplicity, sensitivity, specificity, or multiplexing capabilities, accompanied by specific challenges like assay complexity or optimization requirements.
To learn more about ELISA, download our popular ELISA eBook today!