This website uses cookies to ensure you get the best experience on our website.
- Table of Contents

Immunoglobulins, commonly known as antibodies, play a pivotal role in the immune system's defense against pathogens. These specialized glycoproteins are produced by B cells and are essential for recognizing and neutralizing foreign substances, such as bacteria and viruses, through their antigen-binding sites.
In biomedical research, immunoglobulins are indispensable tools with their ability to bind specifically to antigens. Antibodies are utilized to detect and quantify proteins, pathogens, and other biomolecules through applications like ELISA, Western blotting, and immunohistochemistry.
Immunoglobulins are structurally characterized by their Y-shaped appearance, comprising four polypeptide chains: two identical heavy chains and two identical light chains held together by disulfide bonds.
Each isotype, with its distinct structural and functional properties, is selected based on the specific needs of the experiment. For example, IgG antibodies are often chosen for their high affinity and specificity in detecting target proteins in tissue samples, while IgM, due to its pentameric structure, is preferred in agglutination assays. Researchers also exploit subclass differences, such as IgA's stability in mucosal environments for studying mucosal immunity or IgE for investigating allergic responses and parasite infections.
Isotypes refer to the different classes of immunoglobulins, each with distinct biological properties suited to specific immune responses. The primary differences lie in the structure of their heavy chains and their functional roles within the immune system.
In humans, antibodies are categorized into five isotypes: IgG, IgA, IgM, IgE, and IgD.

Function: IgG is predominantly found in the bloodstream and tissues. It is the most abundant antibody in circulation, comprising about 75% of total antibodies in the bloodstream. It plays a crucial role in providing long-term immunity against pathogens by neutralizing toxins and facilitating phagocytosis. IgG is also capable of crossing the placenta, providing passive immunity to the fetus.
Structure: IgG is composed of two identical heavy chains (gamma chains) and two identical light chains (kappa or lambda chains) linked together by disulfide bonds.
Size: IgG molecules are monomeric, meaning they consist of a single Y-shaped unit.
Research use: IgG is widely used in Western blotting, immunohistochemistry, and ELISA due to its high specificity and affinity for antigens, making it ideal for detecting and quantifying proteins in various samples. You will be able to find a wide range of primary antibodies of this isotype at Boster Bio.
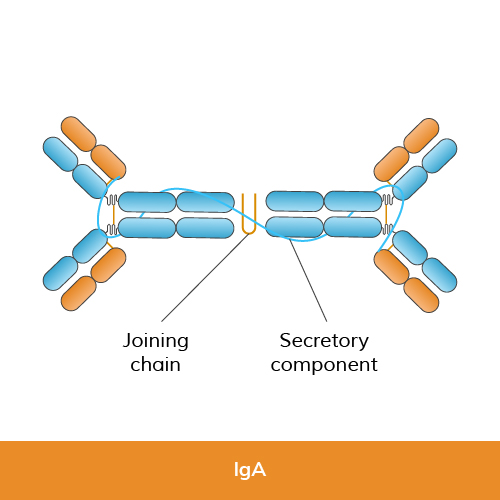
Function: IgA is primarily found in mucosal linings, such as the respiratory and gastrointestinal tracts, as well as in saliva, tears, and breast milk. It plays a key role in mucosal immunity by preventing pathogens from adhering to mucosal surfaces and facilitating their removal.
Structure: IgA has two subclasses, IgA1 and IgA2, each with distinct heavy chain structures. Both subclasses consist of two heavy chains (alpha chains) and two light chains (kappa or lambda chains).
Size: IgA can exist as monomers or dimers, where two IgA molecules are joined together by a Joining (J) chain and secretory component.
Research use: IgA is utilized in studies of mucosal immunity and secretory pathways, often in ELISA and immunofluorescence, to investigate immune responses in mucosal tissues and secretions.
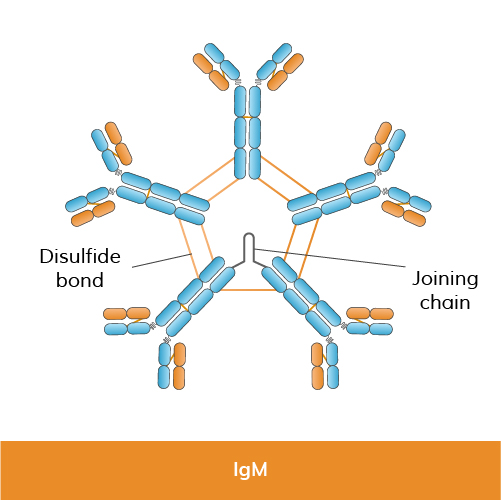
Function: IgM is the largest antibody in terms of size and is the first antibody produced in response to an infection (primary immune response). It exists mainly as a pentamer (five units joined together) in its secreted form, which enhances its ability to bind multiple antigens simultaneously (binding avidity). IgM is crucial for the initial immune response and is often associated with the clearance of pathogens during the early stages of infection.
Structure: IgM is composed of five units—four identical heavy chains (mu chains) and four identical light chains (kappa or lambda chains)—arranged in a pentameric structure.
Size: IgM molecules are large and pentameric, allowing them to bind multiple antigens simultaneously.
Research use: IgM is employed in agglutination assays and early immune response studies because of its pentameric structure, which provides strong binding to multivalent antigens.

Function: IgE is present in small amounts in the bloodstream and is primarily involved in allergic reactions and defense against parasitic infections. It triggers the release of histamine and other chemicals from mast cells and basophils, leading to allergic symptoms like inflammation and bronchoconstriction.
Structure: IgE consists of two identical heavy chains (epsilon chains) and two identical light chains (kappa or lambda chains), similar to other immunoglobulin classes.
Size: IgE molecules are monomeric.
Research use: IgE is used in allergy research and studies of parasitic infections, often in ELISA, to measure and characterize immune responses related to allergic reactions and parasite defenses.
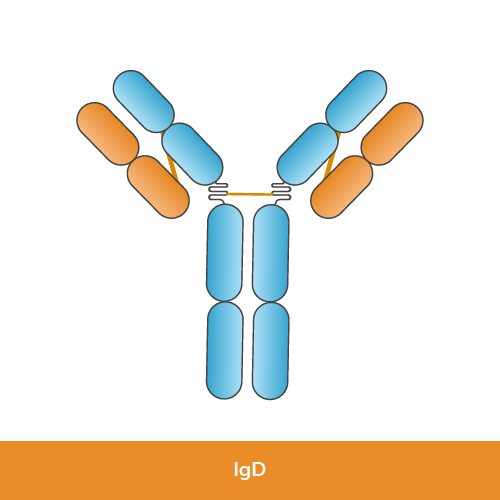
Function: IgD is found in small amounts in the bloodstream and is mainly present on the surface of immature B-lymphocytes and in mucosal areas. Its exact function is still under research, but it is believed to play a role in the activation of B cells.
Structure: IgD has two identical heavy chains (delta chains) and two identical light chains (kappa or lambda chains).
Size: IgD molecules are monomeric.
Research use: IgD is less commonly used in research but is studied in the context of B cell development and activation, primarily through flow cytometry and immunoprecipitation techniques.
These immunoglobulin isotypes vary in their distribution, roles in immune responses, and structural compositions. Below, we’ve provided a comparison table summarizing the immunoglobulin isotypes and their subclasses, highlighting their structural characteristics, size, and primary functions.
| Isotype | Subclasses | Structure | Size | Function and Location |
|---|---|---|---|---|
| IgG | IgG1, IgG2, IgG3, IgG4 | Two gamma (γ) heavy chains + two light chains | Monomeric | Long-term immunity, crosses placenta, opsonization, complement activation |
| IgA | IgA1, IgA2 | Two alpha (α) heavy chains + two light chains | Monomeric/dimer | Mucosal immunity, found in mucosal linings, saliva, tears, breast milk |
| IgM | N/A | Five mu (μ) heavy chains + five light chains | Pentameric | First antibody in primary immune response, large size aids in antigen binding |
| IgE | N/A | Two epsilon (ε) heavy chains + two light chains | Monomeric | Allergic reactions, defense against parasites, binds to mast cells and basophils |
| IgD | N/A | Two delta (δ) heavy chains + two light chains | Monomeric | Found on surface of B cells, role in B cell activation, exact function still under study |

The subclasses within each immunoglobulin isotype differ primarily in their structural characteristics and functional roles within the immune system. Here’s a breakdown of the differences among the subclasses for IgG and IgA:
| Isotype | Structure | Biological Functions | Distribution and Function |
|---|---|---|---|
| IgG | IgG1, IgG3, IgG4: These subclasses have relatively similar structures with variations in their constant (C) regions. | IgG1: Predominant in secondary immune responses, effective in opsonization (marking pathogens for destruction by phagocytes) and complement activation. | IgG1 and IgG3: Found in higher concentrations in serum and tissues, involved in defense against bacterial and viral infections. |
| IgG2: Structurally distinct with longer hinge regions and differences in glycosylation patterns compared to IgG1 and IgG3. | IgG2: Less effective in complement activation but plays a crucial role in opsonization against encapsulated bacteria. | IgG2: More prevalent in mucosal areas and less abundant in serum. | |
| IgG3: Has a shorter half-life but is highly effective in activating complement and neutralizing toxins. | IgG4: Found in lower concentrations, mainly involved in chronic immune responses and allergic reactions. | ||
| IgG4: Often involved in immune tolerance and is less effective in complement activation and opsonization. | |||
| IgA | IgA1: Longer hinge region, more susceptible to proteolytic cleavage. | IgA1: Predominant in serum and mucosal secretions, involved in defense against pathogens by inhibiting their adherence to mucosal surfaces. | IgA1: More prevalent in serum and plays a crucial role in systemic immune responses. |
| IgA2: Shorter hinge region, more resistant to proteolysis. | IgA2: Found mainly in mucosal secretions, provides broader protection against pathogens due to enhanced resistance to proteolysis. | IgA2: Predominantly found in mucosal secretions (e.g., saliva, tears, breast milk) and provides localized immunity at mucosal surfaces. |
Immunoglobulins and their isotypes are a cornerstone of adaptive immunity, showcasing diversity in structure and function. Ongoing research into these antibodies not only enhances our understanding of immune responses but also drives innovations in diagnostic tools and therapeutic interventions.
As essential tools in biomedical research, scientists require antibodies, or immunoglobulins, to identify and quantify proteins, pathogens, and other biomolecules when performing ELISA, WB, IHC, and more applications. Each isotype, with its distinct structural and functional properties, is selected based on the specific needs of the experiment. For example, IgG antibodies are often chosen for their high affinity and specificity in detecting target proteins in tissue samples, while IgM, due to its pentameric structure, is preferred in agglutination assays. Researchers also exploit subclass differences, such as IgA's stability in mucosal environments for studying mucosal immunity or IgE for investigating allergic responses and parasite infections.
At Boster Bio, we specialize in high-quality primary antibodies validated for specificity, affinity, and several research applications, including WB, IHC, IF/ICC, ELISA, and flow cytometry. With over 20,000 antibodies in our catalog, you can find primary antibodies with high specificity and affinity for your research.