This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
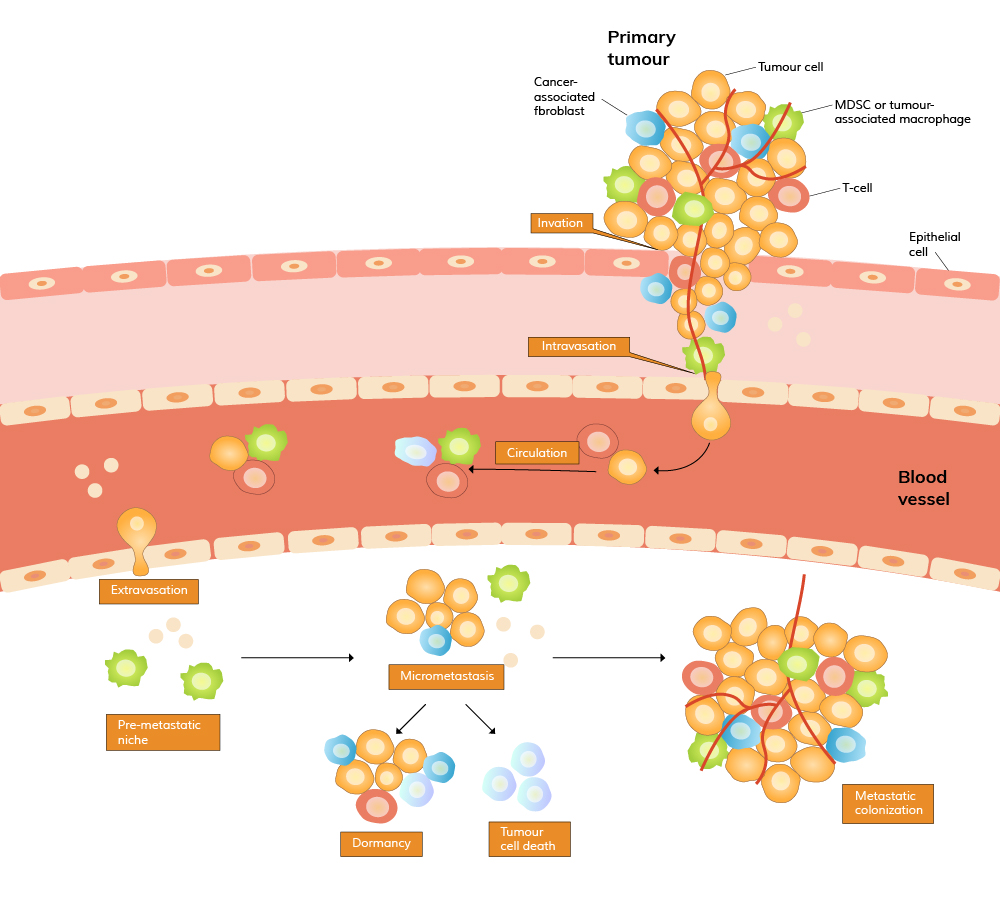
Recent studies have shown that the microenvironment of the target organ, often referred to as the "pre-metastatic niche," plays a significant role in determining where and how tumor cells will establish secondary growth. Factors such as inflammation, immune cell recruitment, and the remodeling of extracellular matrix (ECM) components create a favorable environment for metastatic cells to thrive. Understanding these processes is crucial for developing therapies that can interrupt the metastatic cascade at various stages, potentially inhibiting the spread of cancer before it becomes life-threatening.
Breast Cancer Metastasis
Detecting and managing breast cancer metastasis is particularly critical, as metastasis accounts for 90% of deaths in breast cancer patients despite advances in screening and treatment. Breast cancer metastasis typically involves the spread of malignant cells to bones, lungs, liver, and brain, leading to significant dysfunction in these organs. For instance, bone metastasis can result in severe pain and fractures, while brain metastasis may lead to neurological deficits and cognitive decline. The impact of such metastases on a patient's quality of life and survival cannot be overstated, making the detection and inhibition of breast cancer metastasis a major focus of ongoing research.
Immunohistochemical (IHC) analysis of specific biomarkers, such as HER2, Ki-67, and others, has proven instrumental in detecting breast cancer metastasis. Additionally, circulating tumor cells (CTCs) and cell-free DNA (cfDNA) in the bloodstream are emerging as non-invasive biomarkers that offer valuable insights into breast cancer's progression and potential spread. These techniques not only aid in early detection but also help in understanding the molecular mechanisms underlying breast cancer metastasis, which could lead to the development of targeted therapies.
Anti-HER2 Picoband Anti-Ki67 Picoband
Biomarker Detection
The detection of cancer metastasis is multifaceted and involves identifying various biological processes and signaling molecules associated with the metastatic cascade, including:
Epithelial-Mesenchymal Transition (EMT): During this process, epithelial cells in the primary tumor acquire mesenchymal properties, enhancing their mobility and invasiveness. Markers of EMT can serve as early indicators of metastatic potential.
Secretion of ECM Remodeling Enzymes: Cancer cells often secrete enzymes that degrade the extracellular matrix, facilitating invasion into surrounding tissues. Detection of these enzymes can provide insights into the aggressiveness of the tumor.
Angiogenesis Markers: Cancer cells invade tissues and stimulate the formation of new blood vessels (angiogenesis) to supply nutrients and oxygen. The presence of angiogenesis markers can indicate ongoing metastatic activity.
Quantification of CTCs: The number of CTCs in the blood correlates with the likelihood of metastasis and can be monitored to assess disease progression.
Colonization and Micro-Metastasis Formation: Monitoring the expression of specific genes and proteins involved in colonization can help predict the formation of micro-metastases, often precursors to larger metastatic tumors.
Boster Bio offers a comprehensive range of over 70 antibodies related to cancer metastasis and more than 200 breast cancer markers, providing researchers with the tools needed to investigate these critical processes. Here are some of them:
Inhibiting cancer metastasis is as crucial as detecting it, and strategies are often aligned with the steps of the metastatic process described above. Research has shown that inhibiting EMT, blocking ECM remodeling enzymes, and targeting angiogenesis can significantly reduce the spread of cancer. Antibodies against key proteins involved in these pathways, such as those targeting VEGF, MMPs, and EMT markers, are being developed and tested for their potential to inhibit metastasis.
In the context of breast cancer, targeting specific pathways involved in cell migration and invasion, such as the PI3K/AKT/mTOR pathway, has shown potential in preclinical studies. Additionally, novel biomarkers are being explored to identify high-risk patients who may benefit from more aggressive inhibition strategies. Combining these approaches with traditional therapies, such as chemotherapy and radiation, offers a more comprehensive method to inhibit metastasis and improve patient outcomes.
Anti-VEGF Picoband Anti-HER2 Picoband
We have compiled some of the latest research on inhibiting breast cancer cell metastasis at the cellular level. Hope the unique strategies in these papers will inspire you.




